Taxol Paper
advertisement

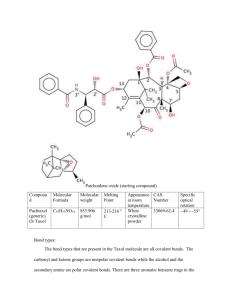
Taxol was discovered in 1967 by Dr. Monroe E. Wall and Dr. Mansukh C. Wani. Working in the Research Triangle Institute, they isolated the compound from the bark of the Pacific Yew tree, Taxus brevifolia. Taxol, or paclitaxel, is a plant alkaloid (Paclitaxel, 2010). Plants use alkaloids to protect themselves from predators. Some are toxic, while others have such a horrible taste that the animal won’t eat it again. Some alkaloids are similar to human neurotransmitters, and have medicinal value (Plant, 2010). Paclitaxel in particular was found to be a good anti-tumor drug when tested in rodents. In December 1992, the FDA approved Taxol as a treatment for ovarian cancer. In April 1994, it was approved as a treatment for breast cancer as well (Taxus, 2010). With the use of Taxol growing, supply became a problem. In order to make enough Taxol to treat only one patient, several trees had to be used. This would eventually destroy the population of the pacific yew tree, so research began to find a way to synthesize the drug without destroying more trees. Because it is a popular landscape plant, a plan was devised to take the clippings from the nurseries that grow them. In these clippings, a precursor to Taxol was isolated. In 1994, Taxol was successfully made through a process known as semi-synthesis (Taxus, 2010). Figure 1: Structure of Taxol (Paclitaxel) (O=Chem, 2010) Taxol, in its pure form, exists as an off-white powder that melts at 198-203 ℃. Its IUPAC name is “Benzenepropanoic acid, beta - (benzoylamino) - alpha - hydroxy -, 6, 12b - bis(acetyloxy) - 12 (benzoyloxy) - 2a, 3, 4, 4a, 5, 6, 9, 10, 11, 12, 12a - , 12b - dodecahydro - 4, 11 - dihydroxy - 4a, 8, 13, 13 - tetramethyl - 5 - oxo - 7,11-methano - 1H - cyclodeca(3, 4)benz(1, 2-b)oxet - 9 - yl ester, (2aR - (2a alpha, 4beta, 4a - beta, 6beta, 9alpha(alphaR*, betaS*), 11alpha, 1 - 2alpha, 12a - alpha, 12b - alpha) (9CI)” (Taxol, 2010). Its molecular formula is C47H51NO14 and its molecular weight is 853.92 grams per mole. Patchoulene oxide (starting compound) Compoun d Molecular Formula Molecular weight Melting Point Appearance CAS at room Number temperature Specific optical rotation Paclitaxel (generic) Or Taxol C47H51NO14 853.906 g/mol 213-216 C White crystalline powder 33069-62-4 - 49 -55 Bond types: The bond types that are present in the Taxol molecule are all covalent bonds. The carbonyl and ketone groups are nonpolar covalent bonds while the alcohol and the secondary amine are polar covalent bonds. There are three aromatic benzene rings in the molecule however the molecule is non-aromatic. While 45 out of the 47 carbon atoms in the molecule are sp3 hybridized the remaining two are sp2 hybridized. Functional groups: There are many functional groups on this molecule. The groups that are duplicated are noted in the parentheses: Phenyl (3), Amide, Alcohol (3), Carbonyl (2), Ester (3), Epoxide ring and an Alkene. Chiral centers: There are eleven chiral centers in the Taxol molecule. In the structure pictured the chiral centers are numbers 1, 2, 3, 4, 5, 7, 8, 10, 13, 2' and 3'. Nine of the eleven chirality centers can be found in what can be called the ABCD ring framework. The A is the hexene ring; the B is the eight carbon ring that together with A comprise an unfused bicyclo ring; C is a six carbon ring that is fused with B and D is referring to the epoxide. Synthesis: The synthesis for Taxol is very long and complicated. The Holton Taxol total synthesis will be the particular synthesis that is elaborated. The Holton synthesis was the first complete synthesis of Taxol that was published in 1994, by Robert A. Holton and colleagues at Florida State University. The reaction is in fact so complex that there is a long list of the groups that were required as they were protecting groups. Benzyloxymethyl, Carbonate, cyclic carbonate, 2-methoxy-2-propyl, tert- butyldimethylsilyl, triethylsilyl, and trimethylsilyl to name a few. The Holton team began with a patchoulol. One reason Holton and his team chose to start the synthesis with patchoulene oxide is the structure of the compound already contains three quarters of the carbons that are needed to comprise the ABCD ring framework. Two important reactions in the total synthesis are enolate oxidation by sulfonyl oxaziridines and a Chan rearrangement. The enolate oxidation happens in the tenth step in the synthesis when a ketone in the number nine position becomes an enolate by oxidation with (+)-camphorsulfonyl oxaziridine. This gives a -hydroxyketone. The importance of this step of the reaction lies in ABCD framework. This sets the stage for the formation of the C ring. The first step in the formation of the C ring is the Chan rearrangement. This is the rearrangement of a carbonate ester in the presence of a strong base (lithium tetramethylpiperidide) to make a -hydroxy ester. There are many more steps in the reaction but few are more important than those that form the ring framework. The mechanism is enantioselective in that synthesizing Taxol from (-)-patchoulene oxide will give the (+)-Taxol enantiomer. The other enantiomer ((-)-Taxol enantiomer) can be obtained by synthesizing Taxol from (-)-borneol. The synthesis is complete, however, initially, Dr. Holton himself claimed that the process was not commercially viable. A big reason for this is that the reaction is an example of linear synthesis, where each subsequent step in the reaction significantly reduces the yield until the final product is exponentially less that all the required reactants. After a series of months the Holton team managed to refine the process, of each step, so that the average chemical yield reached 93 %. Attached is a copy of a summary for the retrosynthesis of Taxol from patchoulene oxide. Taxol is a mitotic inhibitor that is part of a group known as antineoplastic that is taken as a prescription drug that is used with other drugs to treat patients with breast cancer or have returned in six months of treatment and did not respond well to other regiments of treatment, patients with ovarian cancer, lung cancer, some AIDS-related Kaposi's sarcoma patients, prostate, melanoma, esophageal, and including other solid tumors cancers (TAXOLINJECTION, 2010). Taxol is used as a chemotherapeutic agent that depresses the growth by attaching themselves to the cancer cells during the dividing stage causing the cells not to be able to divide resulting in their ability to grow like they should where eventually they die and are unable to spread throughout the rest of the body (Breast Cancer, 2010). Taxol is administered through a fine tube that is inserted into the vein of the patients are for a continuous 24 hours a day, seven days a week, for seven weeks or the cycle your doctor or physician may think is necessary at the time. Taxol should not be given to patients who are pregnant and who have allergic reactions to Taxol as while as similar medications containing castor oil, also having a very low white blood count (Breast Cancer, 2010). While taking Taxol and when treatment has been completed a patient may encounter varies side effects depending on the dosage and the regimen that was given. No all patients will experience these side effects some may experience very few while others could experience more (TAXOL- INJECTION, 2010). Some common side effects are bone marrow decrease in making the appropriate amount of blood cells causing the patient to become anemic also known as having anemia. In addition to the becoming anemic the patient may experience having an irregular heartbeat, slow heart rate, high and low blood pressure, on top of nerve pain and joints two through three days following treatment. Sensations of burning in tingling in hands and feet and may be more at a risk of catching an infection because of your immune system being low from loss of white blood cells being produced. Patients are to avoid any administrates of vaccinations since Taxol may lower you immune system causing the patient to receive the infection they were trying to prevent from catching. Also requires that you avoid close contact with people that are sick and have taken oral polio virus vaccine or that are currently taken it now (TAXOL- INJECTION, 2010). Furthermore Patient may experience some vomiting and nausea which may be mild but other cases not so much, however through it all you must continue the treatment even during these times you may ask your physician if they can prescribe something that will lighten the effects (Paclitaxel-Intravenous Route). Although Taxol has been tested on limited patients from young to adults it has not revealed any differentiation in side effects or problems among the age groups. In deciding if a patient will take Taxol as a form of treatment, the patient must focus on the good it will do in the end; in weighing it to the risk it may cause, but before proceeding to take treatment you must provided your doctor about any allergies you may have from animals, dyes and foods (Paclitaxel-Intravenous Route). In addition to providing your doctor or physician with allergies you may also inform if you have any other medications that are being taken at that time; for taking other medications is not recommended, as well as if you are pregnant or breast feeding for it is not clear if Taxol effects the fetus or can travel to the breast tissue so it is taken as a precautious response. It is very important that during this process you have continues visits with your physician for checking if the treatment is working and is effective and for checking for any unwanted effects of treatment (Paclitaxel-Intravenous Route). In prescribing this treatment if I was a physician or doctor for patients that have ovarian cancer, lung cancer, some AIDS-related Kaposi's sarcoma patients, prostate, melanoma, esophageal, and including other solid tumors cancers. I would administer this as a treatment. Although some of the side effects can cause lifelong problems such as becoming anemic, abnormal heartbeats and live problems. The majority of people do not even experience these major side effects and the outcome after treatment is finished as been very successful for patients. Placing Taxol treatment in the top best treatment known for patients that do have the previous mentioned forms of cancer. Works Cited Clausen, Thomas P., Beverly Johnson, and Jim Wood. "A Mixed Aldol Condensation-Michael Addition Experiment." Journal of Chemical Education 3rd ser. 1996.70 (196): 266-76. ACS Publications. American Chemical Society, 1 Mar. 1996. Web. 26 Feb. 2010. <http://pubs.acs.org/doi/abs/10.1021/ed073p266>. Horwitz, SB. "Taxol (paclitaxel): Mechanisms of Action." Annals of Oncology: Official Journal of the European Society for Medical Oncology 6th ser. 1994.5 (1994): 3-6. National Cnter for Biotechnology Information. National Institutes of Health, 9 Sept. 1994. Web. 2 Feb. 2010. <http://www.ncbi.nlm.nih.gov/pubmed/7865431>. "O=CHem Taxol." University of Southern Maine. Web. 26 Feb. 2010. <http://www.usm.maine.edu/~newton/Chy251_253/Lectures/NaturalProducts/Taxol.html>. "Paclitaxel (Intravenous Route): Precautions - MayoClinic.com." Mayo Clinic Medical Information and Tools for Healthy Living - MayoClinic.com. Web. 07 Mar. 2010. <http://www.mayoclinic.com/health/drug-information/DR601051/DSECTION=precautions->. "Paclitaxel (Taxol." Web. 26 Feb. 2010. <http://www.cancerquest.org/index.cfm?page=526>. "Plant Alkaloids." WAYNE'S WORD. Web. 26 Feb. 2010. <http://waynesword.palomar.edu/ww0703.htm>. "Taxol - Physical Properties and Data." Ross Walker, San Diego Supercomputer Center. Web. 26 Feb. 2010. <http://www.rosswalker.co.uk/taxol/physical.htm>. "TAXOL (paclitaxel) INJECTION(Patient Information Included) Drug Information - Medicine Online." Medical Encyclopedia,Information,Reference,Symptoms,Mental,Women's Health Topics Diseases and Conditions. Web. 27 Feb. 2010. <http://www.medicineonline.com/drugs/T/3558/TAXOL-paclitaxel-INJECTION-PatientInformation-Included.html>. "Taxol, Paclitaxel, Onxal - Chemotherapy Drugs, Chemo Drug Side Effects." Chemotherapy Drugs and Side Effects Information - Chemo Care. Web. 20 Feb. 2010. <http://www.chemocare.com/bio/taxol.asp>. "Taxol Uses." Breast Cancer Home Page. Web. 27 Feb. 2010. <http://breastcancer.emedtv.com/taxol/taxol-uses.html>. "Taxus and Taxol - A Compilation of Research Findings, Special Circular 150-99, Preface." Ohioline. Web. 26 Feb. 2010. <http://ohioline.osu.edu/sc150/sc150_1.html>.