element nutrient quiz answer key
advertisement
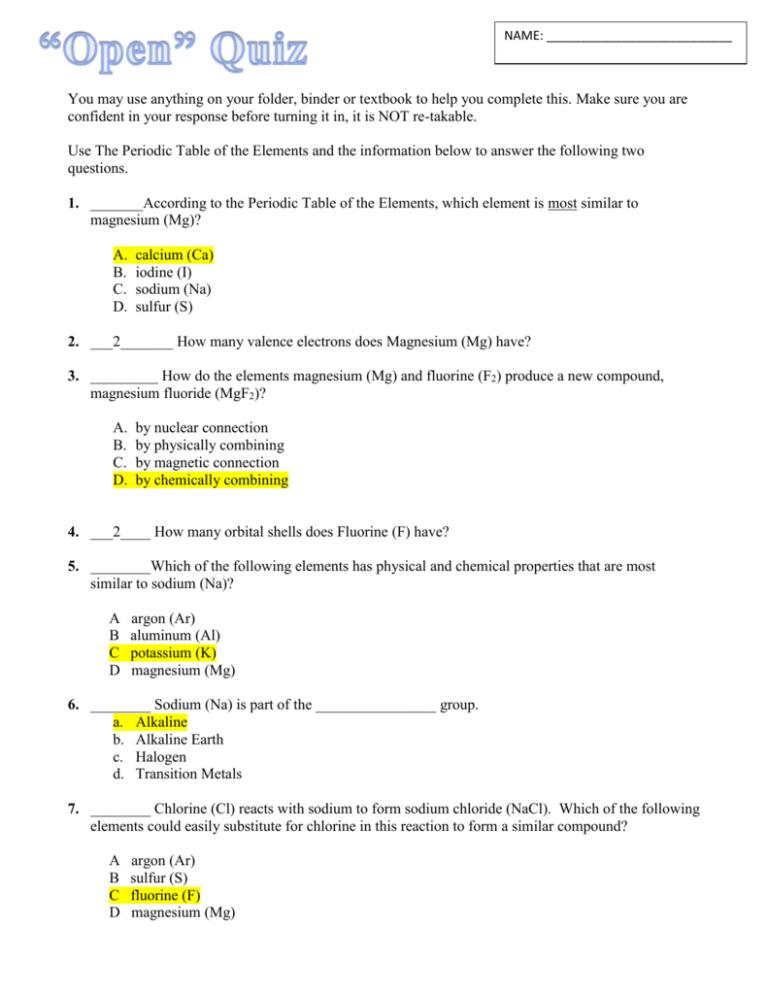
NAME: ___________________________ You may use anything on your folder, binder or textbook to help you complete this. Make sure you are confident in your response before turning it in, it is NOT re-takable. Use The Periodic Table of the Elements and the information below to answer the following two questions. 1. _______According to the Periodic Table of the Elements, which element is most similar to magnesium (Mg)? A. B. C. D. calcium (Ca) iodine (I) sodium (Na) sulfur (S) 2. ___2_______ How many valence electrons does Magnesium (Mg) have? 3. _________ How do the elements magnesium (Mg) and fluorine (F2) produce a new compound, magnesium fluoride (MgF2)? A. B. C. D. by nuclear connection by physically combining by magnetic connection by chemically combining 4. ___2____ How many orbital shells does Fluorine (F) have? 5. ________Which of the following elements has physical and chemical properties that are most similar to sodium (Na)? A B C D argon (Ar) aluminum (Al) potassium (K) magnesium (Mg) 6. ________ Sodium (Na) is part of the ________________ group. a. Alkaline b. Alkaline Earth c. Halogen d. Transition Metals 7. ________ Chlorine (Cl) reacts with sodium to form sodium chloride (NaCl). Which of the following elements could easily substitute for chlorine in this reaction to form a similar compound? A B C D argon (Ar) sulfur (S) fluorine (F) magnesium (Mg) 8. _________Copper (Cu) is a hard, shiny metal. It is not as reactive as sodium, but it does react slowly with other elements, such as oxygen. Which of the following elements has chemical and physical properties similar to copper? a) silver (Ag) b) krypton (Kr) c) calcium (Ca) d) potassium (K) 9. _________ Copper (Cu) has 6 valence electrons. Which element would easily combine with Copper to form a stable compound? a. Lithium (Li) b. Beryllium (Be) c. Boron (B) d. Carbon (C) 10. Which of the following elements is NOT a good conductor of electricity? a. Potassium (K) b. Zinc (Zn) c. Silicon (Si) d. Phosphorus (P) 11. In class, students investigated patterns of elements in The Periodic Table of the Elements. Their teacher pointed out a few properties of some common elements. Helium is the lightest inert (noble) gas in air. Magnesium burns with a bright white flame. Gold and silver are dense metals. Table salt (sodium chloride) is a combination of two reactive elements. Some properties of common elements are shown in the data table below. PROPERTIES OF SOME COMMON ELEMENTS Atomic State of Matter at Room Element Symbol Number Temperature Helium He 2 Gas Chlorine Cl 17 Gas Gold Au 79 Solid Magnesium Mg 12 Solid Silver Ag 47 Solid Sodium Na 11 Solid _______ Which of these elements is a highly reactive metal like sodium (Na)? A. B. C. D. silver (Ag) helium (He) chlorine (Cl) magnesium (Mg) 12. __________ Which element has the same number of valence electrons as Silicon (Si)? a. Sodium (Na) b. Calcium (Ca) c. Tin (Sn) d. Iodine (I) 13. ____47______ How many protons does Silver (Ag) have? 14. _________ How are Sodium (Na) and Lithium (Li) related? a. They both have the same number of electrons b. They both have the same number of valence electrons c. They both have the same number of orbital shells d. They both have the same number of protons 15. _________Which of the following elements have a full outer shell of electrons? a. Elements on the far right of the periodic table b. Elements on the far left of the periodic table c. Elements at the top of the periodic table d. Elements at the bottom of the periodic table 16. __________All three nutrients, carbohydrates, proteins, and lipids are composed of the same elements including: a. Carbon, hydrogen, and nitrogen b. Carbon, hydrogen, and oxygen c. Carbon, nitrogen, and oxygen d. Carbon, potassium, and oxygen 17. _________Which nutrient will yield the most energy when metabolized? a. Fats b. Proteins c. Carbohydrates d. Vitamins and minerals 18. _________Which nutrient functions to transport materials, fight infection, allow muscles to move and provide energy? a. Fats b. Proteins c. Carbohydrates d. Vitamins and minerals 19. ________ Which nutrient(s) is/are made by living things or absorbed by plants and is a helper molecule for many chemical reactions? a. Fats b. Proteins c. Carbohydrates d. Vitamins and minerals 20. _______ Which element is not carbon based? a. Fats b. Proteins c. Vitamins d. Minerals







