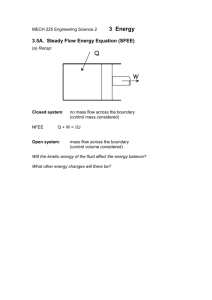
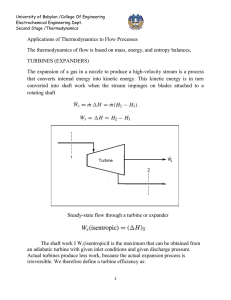
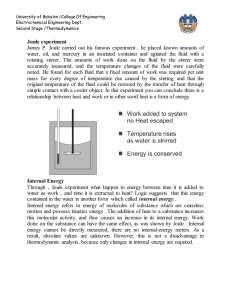
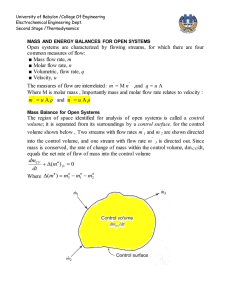
Document 12919583
advertisement
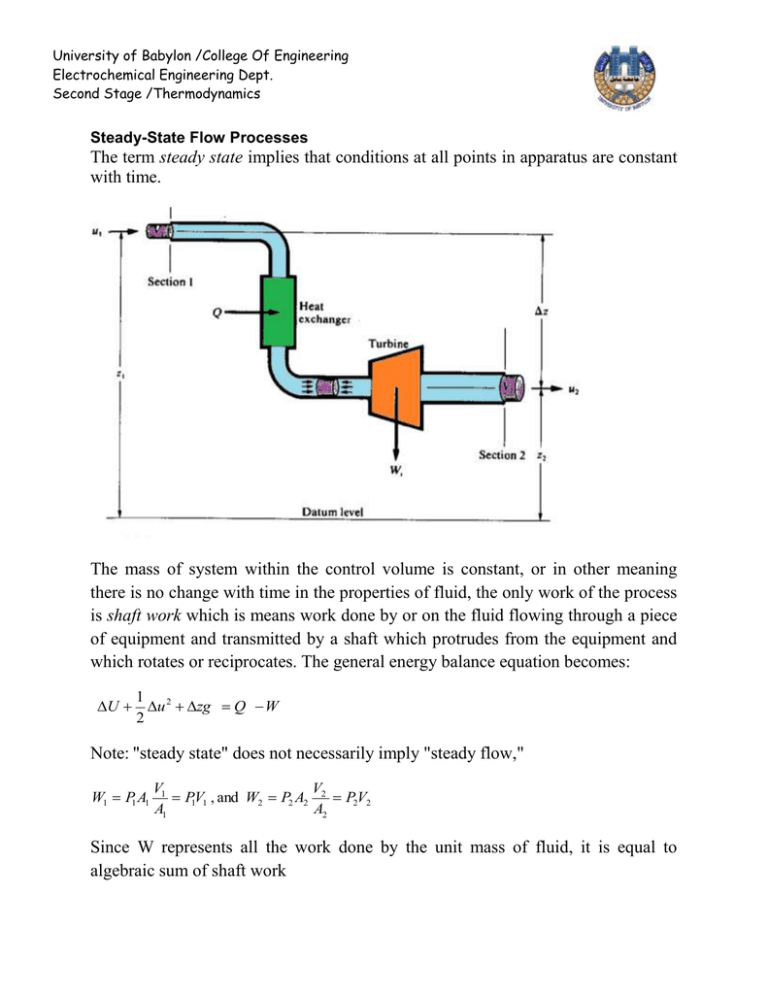
University of Babylon /College Of Engineering Electrochemical Engineering Dept. Second Stage /Thermodynamics Steady-State Flow Processes The term steady state implies that conditions at all points in apparatus are constant with time. The mass of system within the control volume is constant, or in other meaning there is no change with time in the properties of fluid, the only work of the process is shaft work which is means work done by or on the fluid flowing through a piece of equipment and transmitted by a shaft which protrudes from the equipment and which rotates or reciprocates. The general energy balance equation becomes: U 1 2 u zg Q W 2 Note: "steady state" does not necessarily imply "steady flow," W1 P1 A1 V1 V P1V1 , and W2 P2 A2 2 P2V2 A1 A2 Since W represents all the work done by the unit mass of fluid, it is equal to algebraic sum of shaft work University of Babylon /College Of Engineering Electrochemical Engineering Dept. Second Stage /Thermodynamics W WS P2V2 P1V1 U 1 u 2 zg Q WS P2V2 P1V1 2 U ( PV ) H 1 u 2 zg Q WS 2 u 2 zg Q Ws 2 , because H U (PV ) But in many applications, kinetic- and potential-energy terms are omitted, because they are negligible compared with other terms. H Q Ws