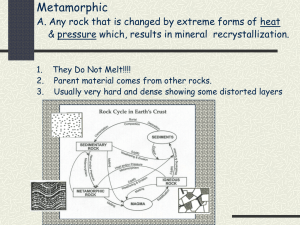
Text-Manual - WordPress.com
advertisement

Unity University Faculty of Engineering Department of Mining Engineering GENERAL GEOLOGY (Geol 2081) By: Tadesse Alemu Director Basic Geoscience Mapping Directorate Geological Survey of Ethiopia October 2012 Addis Ababa 1. INTRODUCTION 1.1. Scope in Mining Engineering Mining Engineering is an engineering discipline that involves the practice, the theory, the science, the technology, and application of extracting and processing minerals from a naturally occurring environment. It also includes processing minerals for additional value. Mining engineers are involved in the mineral discovery stage by working with geologists to identify a mineral reserve. The first step in discovering an ore body is to determine what minerals to test for. Geologists and engineers drill core samples and conduct surface surveys searching for specific compounds and ores. For example, a mining engineer and geologist may target metallic ores such as galena for lead or chalcopyrite for copper. A mining engineer may also search for a non-metal such as phosphate, quartz, or coal. The function of the Mining Engineer is to apply knowledge of pertinent scientific theory, engineering fundamentals, and improved technology to recover natural resources. Mining is a world-wide activity involving the extraction of nonmetallic, metal ores of all kinds, and solid fuels and energy sources such as coal and nuclear material. In addition to mineral extraction, the skills of Mining Engineers are also needed in a variety of fields where the earth's crust is utilized. The construction industry, with its requirements of developing roads, railroads, tunnels, and underground chambers, and the hazardous waste disposal industry are examples of such applications. These are rapidly expanding needs, with a shortage of competent people; the mining engineer is well qualified to meet these needs. The importance of ecological and environmental planning is recognized and given significant attention in all aspects of the mining engineering curriculum. 1.2. Branches of Geology Geology is the study of the Earth. It includes not only the surface process which have shaped the earth's surface, but the study of the ocean floors, and the interior of the Earth. It is not only the study of the Earth as we see it today, but the history of the Earth as it has evolved to its present condition. Geology is divided into several fields, which can be grouped under the major headings of Physical and Historical geology. Physical geology includes: Mineralogy, the study of the chemical composition and structure of minerals; Petrology, the study of the composition and origin of rocks; Geomorphology, the study of the origin of landforms and their modification by dynamic processes; Geochemistry, the study of the chemical composition of earth materials and the chemical changes that occur within the earth and on its surface; Geophysics, the study of the behavior of rock materials in response to stresses and according to the principles of physics; Sedimentology, the science of the erosion and deposition of rock particles by wind, water, or ice; Structural geology, the study of the forces that deform the earth's rocks and the description and mapping of deformed rock bodies; Economic geology, the study of the exploration and recovery of natural resources, such as ores and petroleum; and Engineering geology, the study of the interactions of the earth's crust with human-made structures such as tunnels, mines, dams, bridges, and building foundations. Historical geology deals with the historical development of the earth from the study of its rocks. They are analyzed to determine their structure, composition, and interrelationships and are examined for remains of past life. Historical geology includes: Paleontology, the systematic study of past life forms; Stratigraphy, of layered rocks and their interrelationships; Paleogeography, of the locations of ancient land masses and their boundaries; and Geologic mapping, the superimposing of geologic information upon existing topographic maps. 1.3. The Planet Earth Early History of the Earth The earth and the rest of the solar system were formed about 4.57 billion years ago from an enormous cloud of fragments of both icy and rocky material which was produced from the explosions (super novae) of one or more large stars. It is likely that the proportions of elements in this material were generally similar to those shown below (Fig. 1.1). Although most of the cloud was made of hydrogen and helium, the material that accumulated to form the earth also included a significant amount of the heavier elements, especially elements like carbon, oxygen, iron, aluminum, magnesium and silicon. Figure 1.1. Elemental abundances in the early universe. As the cloud started to contract, most of the mass accumulated towards the centre to become the sun. Once a critical mass had been reached the sun started to heat up through nuclear fusion of hydrogen into helium. In the region relatively close to the sun - within the orbit of what is now Mars - the heat was sufficient for most of the lighter elements to evaporate, and these were driven outward by the solar wind to the area of the orbits of Jupiter and the other gaseous planets. As a result, the four inner planets - Mercury, Venus, Earth and Mars are "rocky" in their composition, while the four major outer planets, Jupiter, Saturn, Neptune and Uranus are "gaseous". As the ball of fragments and dust that was to eventually become the earth grew, it began to heat up - firstly from the heat of colliding particles - but more importantly from the heat generated by radioactive decay (fission) of uranium, thorium, and potassium (Fig. 1.2). Within a few hundred million years the temperature probably rose to several thousand degrees, hot enough to melt most things. This allowed the materials to be sorted out so that the heavier substances sank towards the centre, and the lighter substances floated towards the surface. Figure 1.2. Heat from radioactive decay over geological time. To begin with, much of the iron and magnesium would have combined with silicon and oxygen to form heavy silicate minerals such as olivine: (Mg,Fe)2SiO4 and pyroxene (Mg,Fe)SiO3. Most of the remaining iron (along with some nickel and sulphur) would have migrated towards the centre - forming a very heavy metallic core. Meanwhile, much of the aluminum, sodium and potassium would have combined with oxygen and silicon to form minerals such as quartz (SiO2) and feldspar (NaAlSi3O8) that would have floated towards the surface to form the crust (Fig. 1.3). We will look more closely at the characteristics of the core, mantle and crust later on. The original material that formed the earth included some hydrogen, oxygen, carbon and nitrogen, and these would have been brought to the surface during volcanic eruptions as molecules such as water, carbon dioxide, methane and nitrogen gas. By around 4 billion years (b.y.) ago it is likely that the earth had an atmosphere rich in carbon dioxide and nitrogen along with lots of water vapor. Initially the earth's surface and atmosphere were probably too hot for the water to come down as rain, but as the crust cooled and hardened it kept most of the earth's internal heat inside, and eventually the atmosphere cooled enough for rain to fall and create bodies of water on the surface. Figure 1.3. Differentiation of the early earth. The early atmosphere was largely composed of carbon dioxide, nitrogen, hydrogensulphide, and probably some ammonia and methane. The elements in these substances particularly the carbon, hydrogen, oxygen and nitrogen (CHON) are fundamental to the formation of the molecules that were the precursors of life and those found in all living organisms. There are numerous ways in which these amino acids and other similar molecules could have been formed. For example, in 1953 it was shown by Stanley Millar that amino acids could have formed as a result of the interaction between an electrical current - such as lightning - and ammonia and water - as long as there was no oxygen. The early atmosphere had no free oxygen. We know that because the minerals in very old rocks show no signs of being oxidized (i.e., formed in the presence of oxygen, which commonly results in a reddish colouration due to the oxidation of iron). Sometime in the period between about 4 billion and 3.5 billion years ago primitive life evolved on the earth. No one knows exactly when, how or in what type of environment. The earliest life forms were very simple uni-cellular blobs, very similar to what we call blue-green algae which aren't algae at all but are blue-green bacteria or cyano-bacteria. They fall into the class of life known as prokaryotes - cells that lack nuclei. Like other bacteria, these organisms thrived in an oxygen-free environment. They flourished in their billions and trillions and they produced oxygen and consumed carbon dioxide. At first, almost all of the oxygen that they produced was used up in chemical reactions - the oxidation of dissolved substances such as iron, and of dead organic matter. Eventually, however, around 2.5 billion years ago, the substances that were available to be oxidized started to get used up, and the oxygen level of the atmosphere began to increase. We can see evidence of this oxygenation in the composition and appearance of the rocks that formed starting about 2.2 billion years ago and continuing to the present day. The concept that the earth's environment has been controlled by life (rather than life being controlled by the environment) - is known as the Gaia Hypothesis – an idea which was first proposed by James Lovelock in the 1970s. The traditional way to look at our planet's environment is that it is largely controlled by the laws of physics and chemistry, and that we and the rest of the life forms here are very lucky to have found such a nice place to live. The Gaia hypothesis, which is a comprehensive theory of the interaction between life and the environment, suggests that life itself has contributed to the creation and maintenance of the environment that supports us today3. The following table shows the compositions of the atmospheres of our neighboring planets and what the earth would be like without life - contrasted with the actual composition of our atmosphere. Compositions of the atmospheres of Venus, Earth and Mars Gas Venus Earth without life Mars Carbon dioxide 96.5% 98% 95% Nitrogen 3.5% 1.9% 2.7% Oxygen trace 0 0 Methane 0 0 0 Average T (°C) 459 290 -53 Pressure (bars) 90 60 .00064 Earth with life 0.03% 79% 21% 1.7 ppm 13 1.00 Geologic Time Scale Geologists have divided up time into two eons, namely: Proterozoic, and Phanerozoic. Most of the rocks exposed at surface are of Phanerozoic age. There are exposed rocks of Proterozoic and Archean age. However, some Geologists have divided up time into four eons, namely: Hadean (Pre-Archean), Archean, Proterozoic, and Phanerozoic . Up until recently the oldest known rock in the world - ~ 4.0 billion years (b.y.) old – was the Acasta Gneiss, situated at the eastern edge of Great Slave Lake. In 2008 some even older rocks were discovered at a place called Nuvvuaglittuq on the eastern shore of Hudson Bay. These rocks are estimated to be ~4.28 b.y. old making them Hadean in age. (O’Neill et al., 2008, Science, V. 321, p. 1828.). Figure 1.4. Geologic time scale. Important facts about the planet earth:• The Earth has evolved (changed) throughout its history, and will continue to evolve. • The Earth is about 4.6 billion years old, human beings have been around for only the past 2 million years. Thus, mankind has been witness to only 0.043% of Earth history. The first multi-celled organisms appeared about 700 million years ago. Thus, organisms have only been witness to about 15% of Earth's history. Humans have the capability to make rapid changes. All construction from houses to roads to dams is effected by the Earth, and thus require some geologic knowledge. All life depends on the Earth for food and nourishment. The Earth is there everyday of our lives. Energy and Mineral resources that we depend on for our lifestyle come from the Earth. At present no other source is available. Geologic Hazards: Earthquakes, volcanic eruptions, hurricanes / cyclones, landslides, could affect us at any time. A better understanding of the Earth is necessary to prepare for these eventualities. The materials that make up the Earth are mainly rocks (including soil, sand, silt, dust). Rocks in turn are composed of minerals. Minerals are composed of atoms. Processes range from those that occur rapidly to those that occur slowly. Examples of slow processes • Formation of rocks • Chemical breakdown of rock to form soil (weathering) • Chemical cementation of sand grains together to form rock (diagenesis) • Recrystallization to rock to form a different rock (metamorphism) • Construction of mountain ranges (tectonism) • Erosion of mountain ranges Examples of faster processes • Beach erosion during a storm. • Construction of a volcanic cone • Landslides (avalanches) • Dust Storms • mudflows 2. COMPOSITION AND STRUCTURE OF THE SOLID EARTH Although we can't see into the earth, and the deepest drill-hole is only 13 km, we have a reasonably good picture of its internal structure and composition. Firstly, we can observe material which has been pushed up to surface from great depth - including parts of the ocean floor, and kimberlitic material (A kimberlite is a volcanic eruption with a source deep within the mantle (as opposed to the upper part of the crust for typical volcanoes). Kimberlitic magma travels quickly to the earth's surface and, in some cases, passes through a zone in which diamonds are stable. If some of these diamonds are incorporated into the kimberlite, and if they survive the journey to the surface, a diamond-bearing deposit may form deep within the mantle, the rocks in which diamonds are found. Secondly, we can observe meteorites, most of which are thought to be parts of broken up planets or planetesimals (little planets). Most importantly, however, we can study and understand seismic waves. Seismic waves are physical disturbances in a body of rock caused by earthquakes or artificial explosions - which travel through the rock like a wave across a body of water. They are divided into two types: i). P (Primary, compressional or push) waves - like a coil spring (or slinky) ii). S (Secondary or shear) waves - like a piece of rope which has been flicked P-waves are faster then S-waves4. P-waves will travel through a liquid, while S-waves will not. All waves are bent (refracted) at a boundary between rocks of different composition or physical state (e.g., solid versus liquid). Some important discoveries about the internal characteristics of the earth were made in the early 1900's. For one thing, it was noted that S waves from a large earthquake could not be detected beyond 103° away from the source. This was interpreted as indicating that at least part of the core must behave as a liquid [Figure 10.26]. While P-waves are transmitted through a liquid medium, there is also a P-wave shadow zone extending from 103 to 143° away from the source of a seismic event. Based on an understanding of the refraction of seismic waves, this is also consistent with the idea that part of the core is liquid. Another important discovery based on seismic data was that there is a significant difference in the seismic velocity - and hence geological properties - between the crust and the underlying material (the mantle). Measurements made at different distances from earthquake epicentres showed that seismic waves that travelled downward, and then were refracted laterally actually moved more quickly than those that just travelled laterally. The dividing line between the crust and the mantle is known as the Mohoroviçic Discontinuity, or Moho, after the Yugoslavian who made this discovery. Figure 2.1. Structure of the solid earth. 2.1. The core The core has a total radius of 3486 km - and although it comprises only one-sixth of the volume of the earth, it represents almost one-third of its mass. It is predominantly made up of metallic iron along with some nickel and smaller amounts of sulphur and oxygen the proportion of iron increasing towards the centre. The outer core is liquid, while the inner core (which is actually hotter) is solid. This apparent anomaly can be explained partly by the differences in their composition, but mostly by the difference in pressure. The pressure within the inner core is too great for it to exist as a liquid. The liquid outer part of the core is in motion - and it is this motion - of a material that conducts electricity - that gives the earth its magnetic field. 2.2. The Mantle The mantle has a total thickness of 2885 km and comprises more than 80% of earth's volume (the upper part has the composition of the rock peridotite - which includes the minerals olivine and pyroxene). The temperature increases towards the base of the mantle, but not as much as expected - so we conclude that there must be convection within the mantle to transmit some of the internal heat out towards the surface. It is assumed, therefore, that the mantle behaves in a 'plastic' way. We know that it is essentially solid because it can transmit S-waves, but it will 'flow' in response to longterm stress. The uppermost 100 km of the mantle is solid, and this zone, along with the crust, is known as the lithosphere. The lithosphere does not participate in the convection, and thus the temperature gradient in this zone is quite steep. Immediately below the lithosphere - from 100 km to 200 km - there is a layer of partial melting - also called the low-velocity zone because seismic waves are slowed within it (see figure below). As we will see later, the existence of this partially liquid layer is important to the mechanics of plate tectonics. The low-velocity zone makes up part of the asthenosphere5 that is a relatively mobile part of the mantle. 2.3. The Earth’s Crust The crust is approximately 5 km thick under the oceans and 30 to 60 km thick on the continents. The average global thickness is about 20 km, which represents 0.3% of the total radius of the earth. This is roughly equivalent to the thinnest layer of skin on the outside of an onion. 3. MINERALS AND ROCKS The Earth is composed of rocks. Rocks are aggregates of minerals. Minerals are composed of atoms. In order to understand rocks, we must first have an understanding of minerals. In order to understand minerals we must have some basic understanding of atoms - what they are and how they interact with one another to form minerals. 3.1. Introduction to rock-forming minerals Definition of a Mineral: Naturally formed it forms in nature on its own, Solid (it cannot be a liquid or a gas), With a definite chemical composition (every time we see the same mineral it has the same chemical composition that can be expressed by a chemical formula), and Characteristic crystalline structure (atoms are arranged within the mineral in a specific ordered manner). Examples Glass - can be naturally formed (volcanic glass called obsidian), is a solid, its chemical composition, however, is not always the same, and it does not have a crystalline structure. Thus, glass is not a mineral. Ice - is naturally formed, is solid, and does have a definite chemical composition that can be expressed by the formula H2O. Thus, ice is a mineral, but liquid water is not (since it is not solid). Halite (salt) - is naturally formed, is solid, does have a definite chemical composition that can be expressed by the formula NaCl, and does have a definite crystalline structure. Thus halite is a mineral. Therefore, a mineral is a naturally occurring, inorganic, solid with a definite composition and a regular internal crystal structure. Atomic Chemistry and Bonding All matter is made up of atoms, and all atoms are made up of three main particles known as protons, neutrons and electrons. As summarized in the following table, protons are positively charged, neutrons are uncharged and electrons are negatively charged. The negative charge of one electron balances the positive charge of one proton. Both protons and neutrons have a mass of 1, while electrons have almost no mass. Elementary particle Electron Proton Neutron Charge -1 +1 0 Mass ~0 1 1 The simplest atom is that of hydrogen, which has one proton and one electron. The proton forms the nucleus of hydrogen, while the electron orbits around it. All other elements have neutrons as well as protons in their nucleus. The positively-charged protons tend to repel each other, and the neutrons help to hold the nucleus together. For most of the 16 lightest elements (up to oxygen) the number of neutrons is equal to the number of protons. For most of the remaining elements there are more neutrons than protons, because with increasing numbers of protons concentrated in a very small space, more and more extra neutrons are needed to overcome the mutual repulsion of the protons in order to keep the nucleus together. The number of protons is the atomic number; the number of protons plus neutrons is the atomic weight. For example, silicon has 14 protons, 14 neutrons and 14 electrons. Its atomic number is 14 and its atomic weight is 28. The most common isotope of uranium has 92 protons and 146 neutrons. Its atomic number is 92 and its atomic weight is 238 (92+146). Electron orbits around the nucleus of an atom are arranged in what we call shells. The first shell can hold only two electrons, while the next shell will hold only eight electrons. Subsequent shells can hold more electrons, but the outermost shell of any atom will hold no more than eight electrons. These outermost shells are generally involved in bonding between atoms, and bonding takes place between atoms that do not have the full complement of eight electrons in their outer shells (or two in the first shell for the very light elements). To be chemically stable, an atom seeks to have a full outer shell (i.e., 8 electrons for most elements, or 2 electrons for the very light elements). This is accomplished by lending, borrowing, or sharing electrons with other atoms. Elements that already have their outer orbits filled are considered to be inert; they do not readily take part in chemical reactions. These noble elements include the gases in the right-hand column of the periodic table: helium, neon, argon etc. Sodium has 11 electrons, 2 in the first shell, 8 in the second, and 1 in the third. Sodium readily gives up this third shell electron, and because it loses a negative charge it becomes positively charged. Chlorine, on the other hand, has 17 electrons, 2 in the first shell, 8 in the second, and 7 in the third. Chlorine readily accepts an eighth electron for its third shell, and thus becomes negatively charged. In changing their number of electrons these atoms become ions - the sodium a positive ion or cation, the chlorine a negative ion or anion. The electronic attraction between these ions is known as an ionic bond. Electrons can be thought of as being transferred from one atom to another in an ionic bond. Common table salt (NaCl) is a mineral composed of chlorine and sodium linked together by ionic bonds. The mineral name for NaCl is halite. An element like chlorine can also form bonds without forming ions. For example two chlorine atoms, which each seek an eighth electron in their outer shell, can share an electron in what is known as a covalent bond, to form the gas Cl2. Electrons are shared in a covalent bond. Carbon has 6 protons and 6 electrons, 2 in the inner shell and 4 in the outer shell. Carbon would need to gain or lose 4 electrons to have a filled outer shell, and this would create too great a charge imbalance for the ion to be stable. On the other hand, carbon can share electrons to create covalent bonds. Each carbon atom shares electrons with adjacent carbon atoms. In the mineral diamond the carbon atoms are linked together in a three-dimensional framework, where every bond is a strong covalent bond. In the mineral graphite the carbon atoms are linked together in a two-dimensional hexagonal framework of covalent bonds. Graphite is soft because the bonding between these sheets is relatively weak. Isotopes are atoms of the same element with differing numbers of neutrons. i.e. the number of neutrons may vary within atoms of the same element. Some isotopes are unstable which results in radioactivity. Example: K (potassium) has 19 protons. Every atom of K has 19 protons. Atomic number of K = 19. Some atoms of K have 20 neutrons, others have 21, and others have 22. Thus atomic weight of K can be 39, 40, or 41. 40K is radioactive and decays to 40Ar and 40Ca. Structure of Atoms Electrons orbit around the nucleus in different shells, labeled from the innermost shell as K, L, M, N, etc. Each shell can have a certain number of electrons. The K-shell can have 2 Electrons, the L-shell, 8, the M-shell 18, N-shell 32. #electrons = 2N2, where N=1 for the K shell, N=2 for the L shell, N=3 for the M shell, etc. A Stable electronic configuration for an atom is one 8 electrons in outer shell (except in the K shell, which is completely filled with only 2 electrons). Thus, atoms often loose electrons or gain electrons to obtain stable configuration. Noble gases have completely filled outer shells, so they are stable. Examples He, Ne, Ar, Kr, Xe, Rn. Others like Na, K loose an electron. This causes the charge balance to become unequal. In fact to become + (positive) charged atoms called ions. Positively charged atoms = cations. Elements like F, Cl, O gain electrons to become (-) charged. (-) charged ions are called anions. The drive to attain a stable electronic configuration in the outermost shell along with the fact that this sometimes produces oppositely charged ions, results in the binding of atoms together. When atoms become attached to one another, we say that they are bonded together. Figure 3.1. Electron configuration of an atom. Types of bonding: Ionic bonding- caused by the force of attraction between ions of opposite charge. Example: Na+1 and Cl-1. Bond to form NaCl (halite or salt). Covalent bonding - Electrons are shared between two or more atoms so that each atom has a stable electronic configuration (completely filled outermost shell) part of the time. Example: H has one electron, needs 2 to be stable. O has 6 electrons in its outer shell, needs 2 to be stable. So, 2 H atoms bond to 1 O to form H2O, with all atoms sharing electrons, and each atom having a stable electronic configuration part of the time. Metallic bonding -- Similar to covalent bonding, except innermost electrons are also shared. In materials that bond this way, electrons move freely from atom to atom and are constantly being shared. Materials bonded with metallic bonds are excellent conductors of electricity because the electrons can move freely through the material. Van der Waals bonding -- a weak type of bond that does not share or transfer electrons. Usually results in a zone along which the material breaks easily (cleavage). A good example is graphite. Several different bond types can be present in a mineral, and these determine the physical properties of the mineral. Crystal Structure Solids having a regular, orderly arrangement of their internal atoms are said to have crystalline structure and are known as crystals. Most solid substances, including rocks and minerals, are made up of aggregates of many small crystals. Crystals are characteristically bounded by flat surfaces, which are large-scale reflection of the internal arrangement of the atoms in the crystal. Study of the arrangement of faces in natural crystals showed that there are six basic groups, each with a characteristic symmetry of the faces (Fig. 3.2). Packing of atoms in a crystal structure requires an orderly and repeated atomic arrangement. Such an orderly arrangement needs to fill space efficiently and keep a charge balance. Since the size of atoms depends largely on the number of electrons, atoms of different elements have different sizes. Crystal structure depends on the conditions under which the mineral forms. Figure 3.2. The six basic systems of crystal symmetry. Polymorphs are minerals with the same chemical composition but different crystal structures. The conditions are such things as temperature (T) and pressure (P), because these affect ionic radii. At high T atoms vibrate more, and thus distances between them get larger. Crystal structure changes to accommodate the larger atoms. At even higher T substances changes to liquid and eventually to gas. Liquids and gases do not have an ordered crystal structure and are not minerals. Increase in P pushes atoms closer together. This makes for a more densely packed crystal structure. Examples: The compound Al2SiO5 has three different polymorphs that depend on the temperature and pressure at which the mineral forms. At high P the stable form of Al2SiO5 is kyanite, at low P the stable from is andalusite, and at high T it is sillimanite. Carbon (C) has two different polymorphs. At low T and P pure carbon is the mineral graphite, (pencil lead), a very soft mineral. At higher T and P the stable form is diamond, the hardest natural substance known. In the diagram, the geothermal gradient (how temperature varies with depth or pressure in the Earth) is superimposed on the stability fields of Carbon. Thus we know that when we find diamond it came from someplace in the Earth where the temperature is greater than 1500oC and the pressure is higher than 50,000 atmospheres (equivalent to a depth of about 170 km). CaCO3 - Low Pressure form is Calcite, High Pressure form is Aragonite Figure 3.3. Polymorphs of Carbon. Ionic Substitution (Solid Solution) Ionic substitution - (also called solid solution), occurs because some elements (ions) have the same size and charge, and can thus substitute for one another in a crystal structure. Examples: Olivines Fe2SiO4 and Mg2SiO4. Fe+2 and Mg+2 are about the same size, thus they can substitute for one another in the crystal structure and olivine thus can have a range of compositions expressed as the formula (Mg, Fe)2SiO4. Alkali Feldspars: KAlSi3O8 (orthoclase) and NaAlSi3O8, (albite) K+1 can substitute for Na+1 Plagioclase Feldspars: NaAlSi3O8 (albite) and CaAl2Si2O8 (anorthite) NaSi+5 can substitutes for CaAl+5 (a complex solid solution). Composition of Minerals The variety of minerals we see depend on the chemical elements available to form them. In the Earth's crust the most abundant elements are as follows: 1. O, Oxygen 45.2% by weight 2. Si, Silicon 27.2% 3. Al, Aluminum 8.0% 4. Fe, Iron 5.8% 5. Ca, Calcium 5.1% 6. Mg, Magnesium 2.8% 7. Na, Sodium 2.3% 8. K, Potassium 1.7% 9. Ti, Titanium 0.9% 10. H, Hydrogen 0.14% 11. Mn, Manganese 0.1% 12. P, Phosphorous 0.1% Note that Carbon (one of the most abundant elements in life) is not among the top 12. Because of the limited number of elements present in the Earth's crust there are only about 3000 minerals known. Only 20 to 30 of these minerals are common. The most common minerals are those based on Si and O: the Silicates. Silicates are based on SiO4 tetrahedron. 4 Oxygens covalently bonded to one silicon atom. Figure 3.4. Ionic radii of ions commonly found in rock-forming minerals. Properties of Minerals Physical properties of minerals allow us to distinguish between minerals and thus identify them, as you will learn in lab. Among the common properties used are: Habit - shape Color Streak (color of fine powder of the mineral) Luster -- metallic, vitreous, pearly, resinous (reflection of light) Cleavage (planes along which the mineral breaks easily) Density (mass/volume) Hardness: based on Mohs hardness scale as follows: 1. Talc 2. Gypsum (fingernail) 3. Calcite (penny) 4. Fluorite 5. Apatite (knife blade) 6. Feldspar (Orthoclase) (glass) 7. Quartz 8. Topaz 9. Corundum 10. Diamond Formation of Minerals Minerals are formed in nature by a variety of processes. Among them are: Crystallization from melt (igneous rocks) Precipitation from water (chemical sedimentary rocks, hydrothermal ore deposits) Biological activity (biochemical sedimentary rocks) Change to more stable state - (the processes of weathering, metamorphism, and diagenesis). Precipitation from vapor. (not common, but sometimes does occur around volcanic vents) Since each process leads to different minerals and different mineral polymorphs, we can identify the process by which minerals form in nature. Each process has specific temperature and pressure conditions that can be determined from laboratory experiments. Example: graphite and diamond, as shown previously. Most minerals are made up of a cation (a positively charged ion) or several cations, and an anion (a negatively charged ion) or an anion group. For example, in the mineral hematite (Fe2O3) the cation is Fe (iron) and the anion is O (oxygen). We group minerals into classes on the basis of their predominant anion or anion group. These include oxides, sulphides, carbonates and silicates, and others. Silicates are by far the predominant group in terms of their abundance within the crust and mantle, and they will be discussed later. Some examples of minerals from the different mineral groups are given below. GROUP Oxides Sulphides Carbonates EXAMPLES hematite (iron-oxide – Fe2O3), corundum (aluminum-oxide Al2O3), water-ice (H2O) galena (lead-sulphide - PbS), pyrite (iron-sulphide – FeS2), chalcopyrite (copper-iron-sulphide – CuFeS2) calcite (calcium-carbonate – CaCO3), dolomite (calcium-magnesiumcarbonate – (Ca,Mg)CO3 Silicates Halides Sulphates Phosphate Native elements quartz (SiO2)*, feldspar (sodium-aluminum-silicate – NaAlSi3O8), olivine (iron or magnesium-silicate - FeSiO4) fluorite (calcium-fluoride – CaF2), halite (sodium-chloride - NaCl) gypsum (calcium-sulphate – CaSO4·H2O), barite (barium-sulphate BaSO4) The most important phosphate mineral is apatite (Ca5(PO4)3(OH)) gold (Au), diamond (C), graphite (C), sulphur (S), copper (Cu) *in quartz the anion is oxygen, and while it could be argued, therefore, that quartz is an oxide, it is always classed with the silicates Oxide minerals have oxygen as their anion, but they exclude those with oxygen complexes such as carbonate (CO3), sulphate (SO4), silicate (SiO2) etc. The most important oxides are the iron oxides hematite and magnetite. Both of these are important ores of iron. Corundum (Al2O3) is an abrasive, but can also be a gemstone in its ruby and sapphire varieties. If the oxygen is also combined with hydrogen to form the hydroxyl anion (OH -) the minerals is known as a hydroxide. Some important hydroxides are limonite and bauxite, which are ores of iron and aluminium. Sulphides are minerals with the S-2 anion, and they include galena (PbS), sphalerite (ZnS), chalcopyrite (CuFeS2) and molybdenite (MoS2), which are the main ores of lead, zinc, copper and molybdenum respectively. Some other sulphide minerals are pyrite (FeS2), pyrrhotite, bornite, stibnite, and arsenopyrite. Sulphates are minerals with the SO4-2 anion, and these include gypsum (CaSO4.2H20) and the sulphates of barium and strontium: barite (BaSO4) and celestite (SrSO4). In all of these cases the cation has a +2 charge which balances the -2 charge on the sulphate ion. Halides are so named because the anions include the halogen elements chlorine, fluorine, bromine etc. Examples are halite (NaCl), sylvite (KCl) and fluorite (CaF2). Carbonates include minerals in which the anion is the CO3-2 complex. The carbonate combines with +2 cations to form minerals such as calcite (CaCO 3), magnesite (MgCO3), dolomite ((Ca, Mg)CO3) and siderite (FeCO3). The copper minerals malachite and azurite are also carbonates. Phosphate minerals the anion is PO4-4. The most important phosphate mineral is apatite (Ca5(PO4)3(OH)). Native minerals include only one element, such as gold, copper, sulphur or carbon. Silicate minerals include the elements silicon and oxygen in varying proportions ranging from SiO2 to SiO4. These are discussed at length below. Silicate Minerals The vast majority of the minerals that make up the rocks of the earth's crust are silicate minerals. These include minerals such as quartz, feldspar, mica, amphibole, pyroxene, olivine, and a great variety of clay minerals. The building block of all of these minerals is the silica tetrahedron, a combination of four oxygen atoms and one silicon atom. These are arranged such that planes drawn through the oxygen atoms describe a tetrahedron (a four-faced object)—which is a pyramid with a triangular base (Fig. 3.5). The bonds in a silica tetrahedron have some of the properties of covalent bonds and some of the properties of ionic bonds. As a result of the ionic character, silicon becomes a cation (with a charge of +4) and oxygen becomes an anion (with a charge of -2), hence the net charge of a silica tetrahedron Si04 is -4. As we will see later, silica tetrahedra are linked together in a variety of ways to form most of the common minerals of the crust. Most minerals are characterized by ionic or covalent bonds or a combination of the two, but one other type of bond which is geologically important is the metallic bond. Elements that behave as metals have outer electrons that are relatively loosely held. When bonds between such atoms are formed these electrons can move freely from one atom to another. A metal can thus be thought of as an array of positively charged nuclei immersed in a sea of mobile electrons. This characteristic accounts for two very important properties of metals: their electrical conductivity and their malleability. Figure 3.5. Silicon-oxygen tetrahedron. Name Nesosilicates Sorosilicates Cyclosilicates Inosilicates Structural Group Independent tetrahedra Two tetrahedra sharing one oxygen Closed rings of tetrahedra each sharing two oxygens Unit SiO4 Si2O7 Example Olivine Melilite Typical Formula (Fe,Mg)2SiO4 Ca2MgSi2O7 (SiO3)n n=3,4,6 Beryl (6-fold) Axinite (4-fold) Benitoite (3-fold) Be3Al2(SiO3)6 Ca2(Mn,Fe2)Al2BO3(SiO3)4(OH) BaTi(SiO3)3 MgSiO3 CaSiO3 (a) Continuous single (SiO3) chains of tetrahedra, each sharing two oxygens Pyroxenes Pyroxenoids Phyllosilicates Tektosilicates (b) Continuous double chains of tetrahedra Si4O11 alternately sharing two and three oxygens Continuous sheets of Si4O10 tetrahedra sharing three oxygens Three-dimensional framework of tetrahedra SiO2 with all four oxygen atoms shared Amphiboles Mg7Si8O22(OH)2 Micas Talc KAl2(Si3Al)O10(OH,F)2 Mg3Si4O10(OH)2 Quartz Feldspars SiO2 KAlSi3O8 Figure 3.6. Silicates structure. (A) Nesosilicates; (B) Sorosilicates; (C) A three-membered ring cyclosilicates; (D) A six-membered ring cyclosilicates; (E) A single-chain Inosilicates. i, viewed along the aaxis; ii viewed along the b-axis; iii, viewed along the c-axis; (F) A ribbon Inosilicates. i, viewed along the aaxis; ii, viewed along the c-axis; (G) A Phyllosilicates. 3.2. Igneous Rocks 3.2.1. Origin of Igneous rocks An igneous rock is any crystalline or glassy rock that forms from cooling of magma. Magma consists mostly of liquid rock matter, but may contain crystals of various minerals, and may contain a gas phase that may be dissolved in the liquid or may be present as a separate gas phase. Magma can cool to form an igneous rock either on the surface of the Earth - in which case it produces a volcanic or extrusive igneous rock, or beneath the surface of the Earth, in which case it produces a plutonic or intrusive igneous rock. At depth in the Earth nearly all magmas contain gas dissolved in the liquid, but the gas forms a separate vapor phase when pressure is decreased as magma rises toward the surface. This is similar to carbonated beverages which are bottled at high pressure. The high pressure keeps the gas in solution in the liquid, but when pressure is decreased, like when you open the can or bottle, the gas comes out of solution and forms a separate gas phase that you see as bubbles. Gas gives magmas their explosive character, because volume of gas expands as pressure is reduced. The composition of the gases in magma is: Mostly H2O (water vapor) with some CO2 (carbon dioxide) Minor amounts of Sulfur, Chlorine, and Fluorine gases The amount of gas in magma is also related to the chemical composition of the magma. Rhyolitic magmas usually have higher dissolved gas contents than basaltic magmas. Types of Magma Types of magma are determined by chemical composition of the magma. Three general types are recognized, but we will look at other types later in the course: 1. Basaltic magma (1000-1200oC) -- SiO2 45-55 wt%, high in Fe, Mg, Ca, low in K, Na 2. Andesitic magma (800-1000oC) -- SiO2 55-65 wt%, intermediate. in Fe, Mg, Ca, Na, K 3. Rhyolitic or Granitic magma (650-800oC) -- SiO2 65-75%, low in Fe, Mg, Ca, high in K, Na Viscosity of Magmas Viscosity is the resistance to flow (opposite of fluidity). Depends on composition, temperature, & gas content. Higher SiO2 content magmas have higher viscosity than lower SiO2 content magmas. Lower Temperature magmas have higher viscosity than higher temperature magmas. Summary Table Magma Type Solidified Volcanic Rock Solidified Plutonic Rock Chemical Composition Temperature Viscosity Gas Content Basaltic Basalt Gabbro 1000 - 1200 oC Low Low Andesitic Andesite Diorite 45-55 SiO2 %, high in Fe, Mg, Ca, low in K, Na 55-65 SiO2 %, intermediate in Fe, Mg, Ca, Na, K 800 - 1000 oC Intermediate Intermediate Rhyolitic Rhyolite Granite 65-75 SiO2 %, low in Fe, Mg, Ca, high in K, Na 650 - 800 oC High High Origin of Magma In order for magmas to form, some part of the Earth must get hot enough to melt the rocks present. Under normal conditions, the geothermal gradient is not high enough to melt rocks, and thus with the exception of the outer core, most of the Earth is solid. Thus, magmas form only under special circumstances. To understand this we must first look at how rocks and mineral melt. As pressure increases in the Earth, the melting temperature changes as well. For pure minerals, there are two general cases. For a pure dry (no H2O or CO2 present) mineral, the melting temperate increases with increasing pressure. For a mineral with H2O or CO2 present, the melting temperature first decreases with increasing pressure. Since rocks mixtures of minerals, they behave somewhat differently. Unlike minerals, rocks do not melt at a single temperature, but instead melt over a range of temperatures. Thus, it is possible to have partial melts from which the liquid portion might be extracted to form magma. The two general cases are: Melting of dry rocks is similar to melting of dry minerals, melting temperatures increase with increasing pressure, except there is a range of temperature over which there exists a partial melt. The degree of partial melting can range from 0 to 100% Melting of rocks containing water or carbon dioxide is similar to melting of wet minerals; melting temperatures initially decrease with increasing pressure, except there is a range of temperature over which there exists a partial melt. Origin of Basaltic Magma Much evidence suggests that Basaltic magmas result from dry partial melting of mantle. Basalts make up most of oceanic crust and only mantle underlies crust. Basalts contain minerals like olivine, pyroxene and plagioclase, none of which contain water. Basalts erupt non-explosively, indicating a low gas content and therefore low water content. The Mantle is made of garnet peridotite (a rock made up of olivine, pyroxene, and garnet). Evidence comes from pieces brought up by erupting volcanoes. In the laboratory we can determine the melting behavior of garnet peridotite. Under normal conditions the temperature in the Earth, shown by the geothermal gradient, is lower than the beginning of melting of the mantle. Thus in order for the mantle to melt there has to be a mechanism to raise the geothermal gradient. Once such mechanism is convection, wherein hot mantle material rises to lower pressure or depth, carrying its heat with it. If the raised geothermal gradient becomes higher than the initial melting temperature at any pressure, then a partial melt will form. Liquid from this partial melt can be separated from the remaining crystals because, in general, liquids have a lower density than solids. Basaltic or gabbroic magmas appear to originate in this way. Origin of Granitic or Rhyolitic Magma Most Granitic or Rhyolitic magma appears to result from wet melting of continental crust. The evidence for this is: Most granites and rhyolites are found in areas of continental crust. When granitic magma erupts from volcanoes it does so very explosively, indicating high gas content. Solidified granite or rhyolite contains quartz, feldspar, hornblende, biotite, and muscovite. The latter minerals contain water, indicating high water content. Origin of Andesitic Magma Average composition of continental crust is andesitic, but if andesite magma is produced by melting of continental crust then it requires complete melting of crust. Temperatures in crust unlikely to get high enough. Andesitic magmas erupt in areas above subduction zones, suggests relation between production of andesite and subduction. One theory involves wet partial melting of subducted oceanic crust. But, newer theories suggest wet partial melting of mantle. Magmatic Differentiation When magma solidifies to form a rock it does so over a range of temperature. Each mineral begins to crystallize at a different temperature, and if these minerals are somehow removed from the liquid, the liquid composition will change. Depending on how many minerals are lost in this fashion, a wide range of compositions can be made. The process is called magmatic differentiation by crystal fractionation. Crystals can be removed by a variety of processes. If the crystals are denser than the liquid, they may sink. If they are less dense than the liquid they will float. If liquid is squeezed out by pressure, then crystals will be left behind. Removal of crystals can thus change the composition of the liquid portion of the magma. Over the years, various processes have been suggested to explain the variation of magma compositions observed within small regions. Among the processes are: 1. Distinct melting events from distinct sources. 2. Various degrees of partial melting from the same source. 3. Crystal fractionation. 4. Mixing of 2 or more magmas. 5. Assimilation/contamination of magmas by crustal rocks. 6. Liquid Immiscibility. 7. Combined process (a combination of one of these) Initially, researchers attempted to show that one or the other of these process acted exclusively to cause magmatic differentiation. With historical perspective, we now realize that if any of them are possible, then any or all of these processes could act at the same time to produce chemical change, and thus combinations of these processes are possible. Still, we will look at each one in turn in the following discussion. Distinct Melting Events One possibility that always exists is that the magmas are not related except by some heating event that caused melting. In such a case each magma might represent melting of a different source rock at different times during the heating event. The possibility of distinct melting events is not easy to prove or disprove. Various Degrees of Partial Melting When a multicomponent rock system melts, unless it has the composition of the eutectic, it melts over a range of temperatures at any given pressure, and during this melting, the liquid composition changes. Thus, a wide variety of liquid compositions could be made by various degrees of partial melting of the same source rock. Crystal Fractionation Liquid compositions can change as a result of removing crystals from the liquid as they form. In all cases, crystallization results in a change in the composition of the liquid, and if the crystals are removed by some process, then different magma compositions can be generated from the initial parent liquid. If minerals that later react to form a new mineral or solid solution minerals are removed, then crystal fractionation can produce liquid compositions that would not otherwise have been attained by normal crystallization of the parent liquid. Bowen's Reaction Series Norman L. Bowen, an experimental Petrologist in the early 1900s, realized this from his determinations of simple 2- and 3-component phase diagrams, and proposed that if an initial basaltic magma had crystals removed before they could react with the liquid, that the common suite of rocks from basalt to rhyolite could be produced. This is summarized as Bowen's Reaction Series (Fig. 3.7). Figure 3.7. Bowen’s Reaction Series. Bowen suggested that the common minerals that crystallize from magmas could be divided into a continuous reaction series and a discontinuous reaction series. The continuous reaction series is composed of the plagioclase feldspar solid solution series. A basaltic magma would initially crystallize a Ca- rich plagioclase and upon cooling continually react with the liquid to produce more Na-rich plagioclase. If the early forming plagioclase were removed, then liquid compositions could eventually evolve to those that would crystallize a Na-rich plagioclase, such as a rhyolite liquid. The discontinuous reaction series consists of minerals that upon cooling eventually react with the liquid to produce a new phase. Thus, as we have seen, crystallization of olivine from a basaltic liquid would eventually reach a point where olivine would react with the liquid to produce orthopyroxene. Bowen postulated that with further cooling pyroxene would react with the liquid, which by this time had become more enriched in H2O, to produce hornblende. The hornblende would eventually react with the liquid to produce biotite. If the earlier crystallizing phases are removed before the reaction can take place, then increasingly more siliceous liquids would be produced. This generalized idea is consistent with the temperatures observed in magmas and with the mineral assemblages we find in the various rocks. We would expect that with increasing SiO2 oxides like MgO, and CaO should decrease with higher degrees of crystal fractionation because they enter early crystallizing phases, like olivines and pyroxenes. Oxides like H2O, K2O and Na2O should increase with increasing crystal fractionation because they do not enter early crystallizing phases. Furthermore, we would expect incompatible trace element concentrations to increase with fractionation, and compatible trace element concentrations to decrease. This is generally what is observed in igneous rock suites. Because of this, and the fact that crystal fractionation is easy to envision and somewhat easy to test, crystal fraction is often implicitly assumed to be the dominant process of magmatic differentiation. Mechanisms of Crystal Fractionation In order for crystal fractionation to operate their must be a natural mechanism that can remove crystals from the magma or at least separate the crystals so that they can no longer react with the liquid. Several mechanisms could operate in nature. Crystal Settling/Floating - In general, crystals forming from magma will have different densities than the liquid. If the crystals have a higher density than the liquid, they will tend to sink or settle to the floor of the magma body. The first layer that settles will still be in contact with the magma, but will later become buried by later settling crystals so that they are effectively removed from the liquid. If the crystals have a lower density in the magma, they will tend to float or rise upward through the magma. Again the first layer that accumulates at the top of the magma body will initially be in contact with the liquid, but as more crystals float to the top and accumulate, the earlier formed layers will be effectively removed from contact with the liquid. Inward Crystallization - Because a magma body is hot and the country rock which surrounds it is expected to be much cooler, heat will move outward away from the magma. Thus, the walls of the magma body will be coolest, and crystallization would be expected to take place first in this cooler portion of the magma near the walls. The magma would then be expected to crystallize from the walls inward. Just like in the example above, the first layer of crystals precipitated will still be in contact with the liquid, but will eventually become buried by later crystals and effectively be removed from contact with the liquid. Filter pressing - this mechanism has been proposed as a way to separate a liquid from a crystal-liquid mush. In such a situation where there is a high concentration of crystals the liquid could be forced out of the spaces between crystals by some kind of tectonic squeezing that moves the liquid into a fracture or other free space, leaving the crystals behind. It would be kind of like squeezing the water out of a sponge. This mechanism is difficult to envision taking place in nature because (1) unlike a sponge the matrix of crystals is brittle and will not deform easily to squeeze the liquid out, and (2) the fractures required for the liquid to move into are generally formed by extensional forces and the mechanism to get the liquid into the fractures involves compressional forces. Filter pressing is a common method used to separate crystals from liquid in industrial processes, but has not been shown to have occurred in nature. Magma Mixing If two or more magmas with different chemical compositions come in contact with one another beneath the surface of the Earth, then it is possible that they could mix with each other to produce compositions intermediate between the end members. If the compositions of the magmas are greatly different (i.e. basalt and rhyolite), there are several factors that would tend to inhibit mixing. Temperature contrast - basaltic and rhyolitic magmas have very different temperatures. If they come in contact with one another the basaltic magma would tend to cool or even crystallize and the rhyolitic magma would tend to heat up and begin to dissolve any crystals that it had precipitated. Density Contrast- basaltic magmas have densities on the order of 2600 to 2700 kg/m3, whereas rhyolitic magmas have densities of 2300 to 2500 kg/m3. This contrast in density would mean that the lighter rhyolitic magmas would tend to float on the heavier basaltic magma and inhibit mixing. Viscosity Contrast- basaltic magmas and rhyolitic magmas would have very different viscosities. Thus, some kind of vigorous stirring would be necessary to get the magmas to mix. Crustal Assimilation/Contamination Because the composition of the crust is generally different from the composition of magmas which must pass through the crust to reach the surface, there is always the possibility that reactions between the crust and the magma could take place. If crustal rocks are picked up, incorporated into the magma, and dissolved to become part of the magma, we say that the crustal rocks have been assimilated by the magma. If the magma absorbs part of the rock through which it passes we say that the magma has become contaminated by the crust. Either of these processes would produce a change in the chemical composition of the magma unless the material being added has the same chemical composition as the magma. In a sense, bulk assimilation would produce some of the same effects as mixing, but it is more complicated than mixing because of the heat balance involved. In order to assimilate the country rock enough heat must be provided to first raise the country rock to its solidus temperature where it will begin to melt and then further heat must be added to change from the solid state to the liquid state. The only source of this heat, of course, is the magma itself. Liquid Immiscibility Liquid immiscibility is where liquids do not mix with each other. We are all familiar with this phenomenon in the case of oil and water/vinegar in salad dressing. We have also discussed immiscibility in solids, for example in the alkali feldspar system. Just like in the alkali feldspar system, immiscibility is temperature dependent. Two important properties of immiscible liquids. 1. If immiscible liquids are in equilibrium with solids, both liquids must be in equilibrium with the same solid compositions. 2. Extreme compositions of the two the liquids will exist at the same temperature. Liquid immiscibility was once thought to be a mechanism to explain all magmatic differentiation. If so, requirement 2, above, would require that siliceous liquids and mafic liquids should form at the same temperature. Since basaltic magmas are generally much hotter than rhyolitic magmas, liquid immiscibility is not looked upon favorably as an explanation for wide diversity of magmatic compositions. Still, liquid immiscibility is observed in experiments conducted on simple rock systems. There are however, three exceptions where liquid immiscibility may play a role. 1. Sulfide liquids may separate from mafic silicate magmas. 2. Highly alkaline magmas rich in CO2 may separate into two liquids, one rich in carbonate, and the other rich in silica and alkalies. This process may be responsible for forming the rare carbonatite magmas. 3. Very Fe-rich basaltic magmas may form two separate liquids - one felsic and rich in SiO2, and the other mafic and rich in FeO. Combined Processes As pointed out previously, if any of these processes are possible, then a combination of the process could act to produce chemical change in magmas. Thus, although crystal fractionation seems to be the dominant process affecting magmatic differentiation, it may not be the only processes. As we have seen, assimilation is likely to accompany by crystallization of magmas in order to provide the heat necessary for assimilation. If this occurs then a combination of crystal fraction and assimilation could occur. Similarly, magmas could mix and crystallize at the same time resulting in a combination of magma mixing and crystal fractionation. In nature, things could be quite complicated. 3.2.2. Mode of occurrence of igneous bodies Eruption of Magma When magmas reach the surface of the Earth they erupt from a vent. They may erupt explosively or non-explosively. Non-explosive eruptions are favored by low gas content and low viscosity magmas (basaltic to andesitic magmas). Usually begin with fire fountains due to release of dissolved gases Produce lava flows on surface and produce Pillow lavas if erupted beneath water. B A Figure 3.13. Types of lava flow. (a) Ropy surface of a pahoehoe flow. (b) aa flow, the left side on the photo is a pahoehoe flow. Explosive eruptions are favored by high gas content and high viscosity (andesitic to rhyolitic magmas). Expansion of gas bubbles is resisted by high viscosity of magma, which results in building of pressure. High pressure in gas bubbles causes the bubbles to burst when reaching the low pressure at the Earth's surface. Bursting of bubbles fragments the magma into pyroclasts and tephra (ash). Cloud of gas and tephra rises above volcano to produce an eruption column that can rise up to 45 km into the atmosphere. A B Figure 3.14. Explosive eruptions producing tephra fall (ash) deposit (a); if eruption column collapses a pyroclastic flow (b) may occur, wherein gas and tephra rush down the flanks of the volcano at high speed. This is the most dangerous type of volcanic eruption. The deposits that are produced are called ignimbrites. Structures and field relationships VOLCANOES Shield volcano – volcanoes that erupt low viscosity magma (usually basaltic) that flows long distances from the vent. Pyroclastic cone or cinder cone – a volcano built mainly of tephra fall deposits located immediately around the vent. Stratovolcano (composite volcano) – a volcano built of interbedded lava flows and pyroclastic material. Crater - a depression caused by explosive ejection of magma or gas. Caldera - a depression caused by collapse of a volcano into the cavity once occupied by magma. Lava Dome - a steep sided volcanic structure resulting from the eruption of high viscosity, low gas content magma, Fissure Eruptions - An eruption that occurs along a narrow crack or fissure in the Earth's surface. Pillow Lava - Lavas formed by eruption beneath the surface of the ocean or a lake. PLUTONS Igneous rocks cooled at depth. Name comes from Greek god of the underworld - Pluto. Dikes are small (<20 m wide) shallow intrusions that show a discordant relationship to the rocks in which they intrude. Discordant means that they cut across preexisting structures. They may occur as isolated bodies or may occur as swarms of dikes emanating from a large intrusive body at depth. Sills are also small (<50 m thick) shallow intrusions that show a concordant relationship with the rocks that they intrude. Sills usually are fed by dikes, but these may not be exposed in the field. Laccoliths are somewhat large intrusions that result in uplift and folding of the preexisting rocks above the intrusion. They are also concordant types of intrusions. Batholiths are very large intrusive bodies, usually so large that there bottoms are rarely exposed. Sometimes they are composed of several smaller intrusions. Stocks are smaller bodies that are likely fed from deeper level batholiths. Stocks may have been feeders for volcanic eruptions, but because large amounts of erosion are required to expose a stock or batholith, the associated volcanic rocks are rarely exposed. RELATIONSHIPS TO PLATE TECTONICS To a large extent the location of igneous bodies is related to plate tectonics. Diverging Plate Boundaries Diverging plate boundaries are mostly beneath the oceans and occur at oceanic ridges. Here, basaltic magma is erupted at the oceanic ridge and is intruded beneath the ridge where it forms new oceanic crust. Only rarely does the oceanic ridge build itself above the oceans surface. One example of where this occurs is the island of Iceland in the northern Atlantic Ocean. Eruptions of magma in Iceland are mostly basaltic. Converging Plate Boundaries Where lithospheric plates converge, oceanic lithosphere subducts beneath either another plate composed of oceanic lithosphere or another plate composed of continental lithosphere. If an oceanic lithospheric plate subducts beneath another oceanic lithospheric plate, we find island arcs on the surface above the subduction zone. These are volcanoes built of mostly andesitic lavas pyroclastic material, although some basalts and rhyolites also occur. If an oceanic plate subducts beneath a plate composed of continental lithosphere, we find continental margin arcs. Again, the volcanoes found here are composed mostly of andesitic lavas and pyroclastics. It is likely that some magmas cool beneath the volcanic arc to form dioritic and granitic plutons. Hot Spots Areas where rising plumes of hot mantle reach the surface, usually at locations far removed from plate boundaries are called hot spots. Because plates move relative to the underlying mantle, hot spots beneath oceanic lithosphere produce a chain of volcanoes. A volcano is active while it is over the vicinity of the hot spot, but eventually plate motion results in the volcano moving away from the plume and the volcano becomes extinct and begins to erode. 3.2.3. Textures of Igneous Rocks The main factor that determines the texture of an igneous rock is the cooling rate (dT/dt) Other factors involved are: The diffusion rate - the rate at which atoms or molecules can move (diffuse) through the liquid. The rate of nucleation of new crystals - the rate at which enough of the chemical constituents of a crystal can come together in one place without dissolving. The rate of growth of crystals - the rate at which new constituents can arrive at the surface of the growing crystal. This depends largely on the diffusion rate of the molecules of concern. In order for a crystal to form in magma enough of the chemical constituents that will make up the crystal must be at the same place at the same time to form a nucleus of the crystal. Once a nucleus forms, the chemical constituents must diffuse through the liquid to arrive at the surface of the growing crystal. The crystal can then grow until it runs into other crystals or the supply of chemical constituents is cut off. All of these rates are strongly dependent on the temperature of the system. First, nucleation and growth cannot occur until temperatures are below the temperature at which equilibrium crystallization begins. Shown below are hypothetical nucleation and growth rate curves based on experiments in simple systems. Note that the rate of crystal growth and nucleation depends on how long the magma resides at a specified degree of undercooling (ΔT = Tm - T), and thus the rate at which temperature is lowered below the crystallization temperature. Three cases are shown. 1. For small degrees of undercooling (region A in the figure 3.8) the nucleation rate will be low and the growth rate moderate. A few crystals will form and grow at a moderate rate until they run into each other. Because there are few nuclei, the crystals will be able to grow to relatively large size, and a coarse grained texture will result. This would be called a phaneritic texture. 2. At larger degrees of undercooling, the nucleation rate will be high and the growth rate also high. This will result in many crystals all growing rapidly, but because there are so many crystals, they will run into each other before they have time to grow and the resulting texture will be a fine grained texture. If the sizes of the grains are so small that crystals cannot be distinguished with a handlens, the texture is said to be aphanitic. 3. At high degrees of undercooling, both the growth rate and nucleation rate will be low. Thus few crystals will form and they will not grow to any large size. The resulting texture will be glassy, with a few tiny crystals called microlites. A completely glassy texture is called holohyaline texture. Two stages of cooling, i.e. slow cooling to grow a few large crystals, followed by rapid cooling to grow many smaller crystals could result in a porphyritic texture, a texture with two or more distinct sizes of grains. Single stage cooling can also produce a porphyritic texture. In a porphyritic texture, the larger grains are called phenocrysts and the material surrounding the phenocrysts is called groundmass or matrix Figure 3.8. A hypothetical nucleation and growth rate curves based on experiments in simple systems. In a rock with a phaneritic texture, where all grains are about the same size, we use the grain size ranges shown below to describe the texture: <1 mm 1 - 5 mm fine grained medium grained 5 - 3 cm coarse grained > 3 cm very coarse grained In a rock with a porphyritic texture, we use the above table to define the grain size of the groundmass or matrix, and this table to describe the phenocrysts: 0.03 - 0.3 mm 0.3 - 5 mm microphenocrysts phenocrysts > 5 mm megaphenocrysts Another aspect of texture, particularly in medium to coarse grained rocks is referred to as fabric. Fabric refers to the mutual relationship between the grains. Three types of fabric are commonly referred to: 1. If most of the grains are euhedral - that is they are bounded by well-formed crystal faces. The fabric is said to be idomorphic granular. 2. If most of the grains are subhedral - that is they bounded by only a few well-formed crystal faces, the fabric is said to be hypidiomorphic granular. 3. If most of the grains are anhedral - that is they are generally not bounded by crystal faces, the fabric is said to be allotriomorphic granular. If the grains have particularly descriptive shapes, then it is essential to describe the individual grains. Some common grain shapes are: Tabular - a term used to describe grains with rectangular tablet shapes. Equant - a term used to describe grains that have all of their boundaries of approximately equal length. Fibrous - a term used to describe grains that occur as long fibers. Acicular - a term used to describe grains that occur as long, slender crystals. Prismatic - a term used to describe grains that show an abundance of prism faces. Other terms may apply to certain situations and should be noted if found in a rock. Vesicular - if the rock contains numerous holes that were once occupied by a gas phase, then this term is added to the textural description of the rock. Glomeroporphyritic - if phenocrysts are found to occur as clusters of crystals, then the rock should be described as glomeroporphyritic instead of porphyritic. Amygdular - if vesicles have been filled with material (usually calcite, chalcedony, or quartz, then the term amygdular should be added to the textural description of the rock. An amygdule is defined as a refilled vesicle. Pumiceous - if vesicles are so abundant that they make up over 50% of the rock and the rock has a density less than 1 (i.e. it would float in water), then the rock is pumiceous. Scoraceous- if vesicles are so abundant that they make up over 50% of the rock and the rock has a density greater than 1, then the rock is said to be scoraceous. Graphic - a texture consisting of intergrowths of quartz and alkali feldspar wherein the orientation of the quartz grains resembles cuneiform writing. This texture is most commonly observed in pegmatites. Spherulitic - a texture commonly found in glassy rhyolites wherein spherical intergrowths of radiating quartz and feldspar replace glass as a result of devitrification. Obicular - a texture usually restricted to coarser grained rocks that consists of concentrically banded spheres wherein the bands consist of alternating light colored and dark colored minerals. Other textures that may be evident on microscopic examination of igneous rocks are as follows: Myrmekitic texture - an intergrowth of quartz and plagioclase that shows small wormlike bodies of quartz enclosed in plagioclase. This texture is found in granites. Ophitic texture - laths of plagioclase in a coarse grained matrix of pyroxene crystals, wherein the plagioclase is totally surrounded by pyroxene grains. This texture is common in diabases and gabbros. Subophitic texture - similar to ophitic texture wherein the plagioclase grains are not completely enclosed in a matrix of pyroxene grains. Poikilitic texture - smaller grains of one mineral are completely enclosed in large, optically continuous grains of another mineral. Intergranular texture - a texture in which the angular interstices between plagioclase grains are occupied by grains of ferromagnesium minerals such as olivine, pyroxene, or iron titanium oxides. Intersertal texture - a texture similar to intergranular texture except that the interstices between plagioclase grains are occupied by glass or cryptocrystalline material. Hyaloophitic texture - a texture similar to ophitic texture except that glass completely surrounds the plagioclase laths. Hyalopilitic texture - a texture wherein microlites of plagioclase are more abundant than groundmass and the groundmass consists of glass which occupies the tiny interstices between plagioclase grains. Trachytic texture - a texture wherein plagioclase grains show a preferred orientation due to flowage and the interstices between plagioclase grains are occupied by glass or cryptocrystalline material. Coronas or reaction rims - often times reaction rims or coronas surround individual crystals as a result of the crystal becoming unstable and reacting with its surrounding crystals or melt. If such rims are present on crystals they should be noted in the textural description. Patchy zoning - This sometimes occurs in plagioclase crystals where irregularly shaped patches of the crystal show different compositions as evidenced by going extinct at angles different from other zones in the crystal. Oscillatory zoning - This sometimes occurs in plagioclase grains wherein concentric zones around the grain show thin zones of different composition as evidenced by extinction phenomena. Moth eaten texture (also called sieve texture)- This sometimes occurs in plagioclase wherein individual plagioclase grains show an abundance of glassy inclusions. Perthitic texture - Exsolution lamellae of albite occurring in orthoclase or microcline. Antiperthitic texture – Exsolution lamellae of orthoclase or microcline occurring in albite. 3.2.4. Classification of Igneous rocks Classification of igneous rocks is one of the most confusing aspects of geology. This is partly due to historical reasons, partly due to the nature of magmas, and partly due to the various criteria that could potentially be used to classify rocks. Early in the days of geology there were few rocks described and classified. In those days each new rock described by a geologist could have shown characteristics different than the rocks that had already been described, so there was a tendency to give the new and different rock a new name. Because such factors as cooling conditions, chemical composition of the original magma, and weathering effects, there is a potential to see an infinite variety of igneous rocks, and thus a classification scheme based solely on the description of the rock would eventually lead to a plethora of rock names. Still, because of the history of the science, many of these rock names are firmly entrenched in the literature, so the student must be aware of all of these names, or at least know where to look to find out what the various rocks names mean. Magmas, from which all igneous rocks are derived, are complex liquid solutions. Because they are solutions, their chemical composition can vary continuously within a range of compositions. Because of the continuous variation in chemical composition there is no easy way to set limits within a classification scheme. There are various criteria that could be used to classify igneous rocks. Among them are: 1. Minerals Present in the Rock (the mode). The minerals present in a rock and their relative proportions in the rock depend largely on the chemical composition of the magma. This works well as a classification scheme if all of the minerals that could potentially crystallize from the magma have done so - usually the case for slowly cooled plutonic igneous rocks. But, volcanic rocks usually have their crystallization interrupted by eruption and rapid cooling on the surface. In such rocks, there is often glass or the minerals are too small to be readily identified. 2. Texture of the Rock. Rock texture depends to a large extent on cooling history of the magma. Thus rocks with the same chemical composition and same minerals present could have widely different textures. In fact we generally use textural criteria to subdivide igneous rocks in to plutonic (usually medium to coarse grained) and volcanic (usually fine grained, glassy, or porphyritic.) varieties. 3. Color. Color of a rock depends on the minerals present and on their grain size. Generally, rocks that contain lots of feldspar and quartz are light colored, and rocks that contain lots of pyroxenes, olivines, and amphiboles (ferromagnesium minerals) are dark colored. But color can be misleading when applied to rocks of the same composition but different grain size. For example granite consists of lots of quartz and feldspar and is generally light colored. But a rapidly cooled volcanic rock with the same composition as the granite could be entirely glassy and black colored (i.e. an obsidian). Still we can divide rocks in general into felsic rocks (those with lots of feldspar and quartz) and mafic rocks (those with lots of ferromagnesium minerals). But, this does not allow for a very detailed classification scheme. 4. Chemical Composition. Chemical composition of igneous rocks is the most distinguishing feature. The composition usually reflects the composition of the magma, and thus provides information on the source of the rock. The chemical composition of the magma determines the minerals that will crystallize and their proportions. A set of hypothetical minerals that could crystallize from a magma with the same chemical composition as the rock (called the Norm), can facilitate comparison between rocks. Still, because chemical composition can vary continuously, there are few natural breaks to facilitate divisions between different rocks. Chemical composition cannot be easily determined in the field, making classification based on chemistry impractical. Because of the limitations of the various criteria that can used to classify igneous rocks, geologists use an approach based on the information obtainable at various stages of examining the rocks. 1. In the field, a simple field based classification must be used. This is usually based on mineralogical content and texture. For plutonic and volcanic rocks, the IUGS system of classification can be used (Figs. 3.9 and 3.10), and for pyroclastic rocks (Fig. 3.11). A B C Figure 3.9. IUGS classification of plutonic rocks. (a) Felsic rocks, (b) Mafic rocks, and (c) Ultramafic rocks. Q Figure 3.10. Classification of volcanic rocks recommended by IUGS. 60 60 Rhyolite Dacite 20 20 Trachyte Latite 35 A 10 (foid)-bearing Trachyte Andesite/Basalt 65 (foid)-bearing Latite Phonolite (foid)-bearing Andesite/Basalt P 10 Tephrite 60 60 (Foid)ites F Figure 3.11. Classification of the pyroclastic rocks. a. Based on type of material. After Pettijohn (1975) Sedimentary Rocks, Harper & Row, and Schmid (1981) Geology, 9, 40-43. b. Based on the size of the material. After Fisher (1966) Earth Sci. Rev., 1, 287298. 2. Once the rocks are brought back to the laboratory and thin sections can be made, these are examined, mineralogical content can be more precisely determined, and refinements in the mineralogical and textural classification can be made. 3. Chemical analyses can be obtained, and a chemical classification, such as the LeBas et al., IUGS chemical classification of volcanic rocks (based on total alkalies [Na2O + K2O] vs. SiO2 (Fig. 3.12). Figure 3.12. IUGS chemical classification of volcanic rocks (based on total alkalies [Na2O + K2O] vs. SiO2. 4. General chemical classification SiO2 (Silica) Content > 66 wt. % - Acid 52-66 wt% - Intermediate 45-52 wt% - Basic < 45 wt % - Ultrabasic Silica Saturation: If magma is oversaturated with respect to Silica then a silica mineral, such as quartz, cristobalite, tridymite, or coesite, should precipitate from the magma, and be present in the rock. On the other hand, if magma is undersaturated with respect to silica, then a silica mineral should not precipitate from the magma, and thus should not be present in the rock. The silica saturation concept can thus be used to divide rocks in silica undersaturated, silica saturated, and silica oversaturated rocks. The first and last of these terms are most easily seen. Silica Undersaturated Rocks - In these rocks we should find minerals that, in general, do not occur with quartz. Such minerals are: o o o o o o Nepheline- NaAlSiO4 Leucite - KAlSi2O6 Forsteritic Olivine - Mg2SiO4 Sodalite - 3NaAlSiO4 Perovskite - CaTiO3 Melanite - Ca2Fe+3Si3O12 Melilite - (Ca,Na)2(Mg,Fe+2,Al,Si)3O7 Thus, if we find any of these minerals in a rock, with an exception that we'll see in a moment, then we can expect the rock to be silica undersaturated. Silica Oversaturated Rocks. These rocks can be identified as possibly any rock that does not contain one of the minerals in the above list. Silica Saturated Rocks. These are rocks that contain just enough silica that quartz does not appear, and just enough silica that one of the silica undersaturated minerals does not appear. Alumina (Al2O3) Saturation After silica, alumina is the second most abundant oxide constituent in igneous rocks. Feldspars are, in general, the most abundant minerals that occur in igneous rocks. Thus, the concept of alumina saturation is based on whether or not there is an excess or lack of Al to make up the feldspars. Note that Al2O3 occurs in feldspars in a ratio of 1 Al to 1 Na, 1K, or 1 Ca: KAlSi3O8 -- 1/2K2O : 1/2Al2O3 NaAlSi3O8 -- 1/2Na2O : 1/2Al2O3 CaAl2Si2O8 -- 1CaO : 1Al2O3 Three possible conditions exist. 1. If there is an excess of Alumina over that required forming feldspars, we say that the rock is peraluminous. This condition is expressed chemically on a molecular basis as: Al2O3 > (CaO + Na2O + K2O). In peraluminous rocks we expect to find an Al2O3-rich mineral present as a modal mineral - such as muscovite [KAl3Si3O10(OH)2], corundum [Al2O3], topaz [Al2SiO4(OH,F)2], or an Al2SiO5- mineral like kyanite, andalusite, or sillimanite. Peraluminous rocks will have corundum [Al2O3] in the CIPW norm and no diopside in the norm. 2. Metaluminous rocks are those for which the molecular percentages are as follows: Al2O3 < (CaO + Na2O + K2O) and Al2O3 > (Na2O + K2O). These are the more common types of igneous rocks. They are characterized by lack of an Al2O3-rich mineral and lack of sodic pyroxenes and amphiboles in the mode. 3. Peralkaline rocks are those that are oversaturated with alkalies (Na2O + K2O), and thus undersaturated with respect to Al2O3. On a molecular basis, these rocks show: Al2O3 < (Na2O + K2O). Peralkaline rocks are distinguished by the presence of Na-rich minerals like aegerine [NaFe+3Si2O6], riebeckite [Na2Fe3+2Fe2+3Si8O22(OH)2], arfvedsonite[Na3Fe4+2(Al,Fe+3)Si8O22(OH)2],or aenigmatite [Na2Fe5+2TiO2Si6O18] in the mode. Alkaline/Subalkaline Rocks One last general classification scheme divides rocks that alkaline from those that are subalkaline. Note that this criterion is based solely on an alkali vs. silica diagram, as shown below. Alkaline rocks should not be confused with peralkaline rocks as discussed above. While most peralkaline rocks are also alkaline, alkaline rocks are not necessarily peralkaline. On the other hand, very alkaline rocks, that are those that plot well above the dividing line in the figure below, are also usually silica undersaturated. Figure 3.12. Diagram showing Alkaline and Subalkaline division. 3.3. Sedimentary Rocks 3.3.1. Nature and Origin of Sedimentary rocks Sedimentary rocks are deposited on or near Surface of Earth by Mechanical or Chemical Processes. Sedimentary rocks are the principal repository for information about the Earth’s past Environment. Depositional environments in ancient sediments are recognized using a combination of sedimentary facies, sedimentary structures and fossils. Based on their origin and composition, sedimentary rocks are classified in to three major classes. 1. Clastic Rocks 2. Chemical Rocks 3. Bioclastic Rocks Clastic rocks • • • • Chemical rocks Sandstones Conglomerates Breccia Shale/mudstones Carbonate rocks Form basically from CaCO3 – both by chemical leaching and by organic source (biochemical) e.g. Limestone; dolomite Bioclastic (organic) rocks Form due to decomposition of organic remains under temperature and pressure e.g. Coal/Lignite etc. Evaporitic rocks These rocks are formed due to evaporation of Saline water (sea water) e.g. Gypsum, Halite (rock salt) 1. Clastic Rocks: Those rocks composed of fragments (clasts) of any pre-existing rocks. The fragments may be of a single mineral (clay minerals, mica grains, quartz grains, feldspar grains …), or may also be fragments of rocks (e.g. Shale clasts, granite pebbles …). Regardless of their origin and composition, such clasts are further classified by their size (see grain size parameter). 2. Chemical Rocks: These rocks are the products of chemical and/or biological precipitation of sediments from chemically saturated water. These includes carbonates (limestone and dolomite), evaporates (halite, gypsum, anhydrite) and chert (SiO2). 3. Bioclastic Rocks: Composed of organic debris such as plants (lignite, coal), of shells (coquina, fossiliferous limestone), and of microorganisms (oilshale, cocoliths, radiolarian earth). CLASTIC ROCKS Formed from broken rock fragments weathered and eroded by river, glacier, wind and sea waves. These clastic sediments are found deposited on floodplains, beaches, in desert and on the sea floors. Clastic rocks are classified by: • Grain Size • Grain Composition • Texture Clastic rocks are classified on the basis of the grain size in to: conglomerate, sandstone, mudstone etc. Conglomerates & Breccias: > 30% gravel (>2 mm) and larger clastic grains (< 5 %) Sandstones: > 50% sand sized (0.062 - 2 mm) clastic grains ( 20 %) Mudstones: > 50% silt (0.062 - 004 mm) and/or clay (< 0.004 mm) ( 65 %) The formation of a clastic sedimentary rock involves three processes: Transportation- Sediment can be transported by sliding down slopes, being picked up by the wind, or by being carried by running water in streams, rivers, or ocean currents. The distance the sediment is transported and the energy of the transporting medium all leave clues in the final sediment of the mode of transportation. Deposition - Sediment is deposited when the energy of the transporting medium becomes too low to continue the transport process. In other words, if the velocity of the The Udden-Wentworth grain-size scale Grain-size (mm) 64-256 Cobble 4-64 Pebble Sediment Gravel 2-4 Granule 1-2 Very Coarse Sand 0.5-1 Coarse Sand 0.25-0.5 Medium Sand 0.125-0.25 0.625-0.125 Sand Fine Sand Very Fine Sand 0.031-0.625 Coarse Silt 0.016-0.031 Medium Silt 0.008-0.016 Fine Silt Silt Very Fine Silt Clay Clay 0.004-0.008 <0.004 transporting medium becomes to low to transport sediment, the sediment will fall out and become deposited. The final sediment thus reflects the energy of the transporting medium. Diagenesis - is the process that turns sediment into rock. The first stage of the process is compaction. Compaction occurs as the weight of the overlying material increases. Compaction forces the grains closer together, reducing pore space and eliminating some of the contained water. Some of this water may carry mineral components in solution, and these constituents may later precipitate as new minerals in the pore spaces. This causes cementation, which will then start to bind the individual particles together. Further compaction and burial may cause recrystallization of the minerals to make the rock even harder. Conglomerate and Breccia Conglomerate and Breccia are clastic rocks consist of clasts greater than 2 mm size (gravel). If rounded clasts; it is conglomerate and if angular clasts it is breccia. A B Figure 3.13. (a) Clasts and matrix (labeled), and iron oxide cement (reddish brown color). Rounded clasts (conglomerate) (b), and angular clasts (breccia) (c). C Sandstones • • • Sandstones ( > 50% sand-sized (0.062 - 2 mm) clastic grains ), Sandstones are classified according to the types of clastic grains present (quartz, feldspar, & lithic fragments) and the presence (wackes) or absence (arenites) of significant fine-grained matrix material (< 0.03 mm), After this subdivision they are described in terms of the types of preserved sedimentary structures, using terms like cross-bedded sandstone and relative maturity using criteria such as degree of sorting, roundness of the clasts, diversity of clast types, etc. Arenites: fine-grained matrix not visible to naked eye (<10-15%). quartz arenite (~ 35 %) : quartz grains 90 %. Rare in the modern environment, but quite common in late Precambrian and Paleozoic. Tend to be relatively mature, and may represent end product of several cycles of erosion, transport, and deposition. Silica cement predominates. synonym = orthoquartzite feldspathic arenite (~ 15 %) : feldspar / (felds + rock frag.) 50 %. commonly developed in granitic terranes and therefore restricted to local basins, but may also develop in cold or arid climates where feldspar is relatively resistant to decomposition, or in areas of high erosion rates. Typically cemented by calcite. synonym = arkose, if felds is K-spar lithic arenite(~ 20 %) : rock fragments / (felds + rock frag.) 50% The most abundant sandstone, as the sand-sized sediment loads of most modern rivers are dominated by lithic clasts. Furthermore, if greywackes are derived from the decomposition of lithic and feldspar clasts, then lithic arenites comprise 50 % of all arenites. Tend to be immature, poorly sorted. Typically cemented by calcite. synonym = subgreywacke Greywacke: sandstone with a fine-grained matrix visible to the naked eye (> 10-15% matrix with < 0.03 mm grain-size). Commonly the presence of this matrix gives the rock a dark grey color. The clastic grains are typically polymictic and commonly relatively angular. The matrix is composed of finely crystalline chlorite and sericite developed during diagenesis, along with silt-size quartz and albite. This fine-grained matrix has reacted with and obliterated the original outline of the clastic grains, acting as the cementing agent. There are two hypotheses for the origin of the matrix: 1. Diagenetically altered silt and clay that were initially present between the coarser sand-sized grains. 2. Diagentically altered lithic, and feldspar, clastic grains of a former lithic arenite. Figure 3.14. Classification of sandstones. Mudstones Mudstones: (> 50% silt (0.062 - 004 mm) and / or clay (< 0.004 mm) • Mudstones are composed of silt-sized quartz and feldspar grains and much smaller clay mineral particles. Depending of the relative proportions of these two types of grains, mudstones range from siltstones to shales, and claystones. • Siltstones can be distinguished from shales and mudstones by biting a piece between your teeth. If it feels "gritty" then it is a siltstone, if it feels smooth or slick, then it is a shale or claystone. • One of the most important features of mud rocks is their color, an indication of their oxidation state and the paleo-environment of their deposition: – Red shales are oxidized and typically represent sub-aerial detritus derived from the continents. They may represent in sub-aerial deposits, but also are formed by continental dust settling into organic-poor deep marine environments. – – Green shales are relatively reduced, and common in the shallow submarine environments depleted in oxygen by the decay of organic matter. Black shales are rich in organic matter and highly reduced, typically deposited in anoxic environments. They sometimes act as source rocks from which oil and gas are released during burial and diagenesis. Figure 3.15. Classification of mudstones. CHEMICAL ROCKS Carbonate sediments These are represented by limestone and dolomite. Limestones They are a non-clastic rock formed either chemically or due to precipitation of calcite (CaCO3) from organisms usually (shell). These remains will result in formation of a limestone. Limestones formed by chemical precipitation are usually fine grained, whereas; in case of organic limestone the grain size vary depending upon the type of organism responsible for the formation. Calcium carbonate exists as the mineral polymorphs calcite and aragonite. Both may form as inorganic precipitates or as biological secretions in the hard parts of numerous organisms. Aragonite does not usually precipitate from fresh water, and is unstable under Earth surface conditions. It is a high-pressure equilibrium carbonate. Because of the charge similarity and ionic radius of Ca+2 and Mg+2 ions, and because of the structure of the calcite lattice, Mg+2 may substitute extensively for Ca2+ in calcite. Hence, those calcites with more than 5% MgCO3 are known as high-Mg calcites. Recent shallow-water tropical and subtropical calcium carbonate deposits are predominantly composed of aragonite and high-Mg calcite, whilst temperate shallow carbonates contain dominantly calcite. Limestones are composed of such components that can be distinguished into four broad groups: i) non-skeletal grains ii) skeletal grains iii) micrite and iv) cement i) Non-skeletal grains: includes peloids, ooids, aggregates, litho- and intraclasts. Peloids are sand sized grains of mud-grade carbonate resulted from different processes. Some classic varieties are known as pellets distinguished by their smaller size and well sorting. Ooids are also sand-sized grains with distinctive concentric coats of carbonate around shell fragments, quartz grains or peloids. Aggregates are sand-sized particles that have been agglutinated to form compound grains. Lithoclasts are recognizable clasts of lithified, pre-existing carbonate sediment dissimilar to its host sediment or to sediments associated with its host. Intraclasts are clasts composed of sediments which are represented either in the host sediment or in associated sediments. ii) Biogenic carbonates: are the main components in most limestones and consist of the remains of calcareous protozoan, metazoans and plants. This calcareous material is broken down by physical, chemical and biological processes with each kind of skeletal or calcareous plant material behaving differently. Most of this biogenic material ends up as disarticulated, abraded and fragmented detrital bioclasts but some, especially the larger skeletons of colonial organisms are calcareous algae, can remain in situ. Such material, commonly encrusted by other organisms or cemented by carbonate cement, forms the framework of some types of reef. iii) Carbonate mud/micrite is a major component of limestones and is also polygenic. Calcareous algae in shallow waters, particularly green algae, are capable of producing vast quantities of aragonite mud as their calcified Three classification schemes are currently used, each with a different emphasis, but the third, that of Dunham, based on texture, is now used more widely. 1. A very simple but often useful scheme divides limestones on the basis of grain size into calcirudite (most grains >2mm), calcarenite (most grains between 2mm and 62um) and calcilutite (most grains <62um). 2. The classification scheme of R.L. Folk based mainly on composition, distinguishes three components: (a) the grains (allochems), (b) matrix, chiefly micrite and (c) cement, usually drusy sparite. An abbreviations for the grains used as prefixes are bio- (referring skeletal components), oo- (for ooids), pel- (for peloids), and intra- (for intraclasts) together with either sparite or micrite, whichever is the dominant. Terms can be combined if two types of grains are dominant in a rock, as in biopelsparite, or bio-oosparite. Terms can be modified to give an indication of coarse grain size, as in biosparrudite, or intramicrudite. 3. Dunham classification of limestones divides the rocks into: grainstone, grains without matrix (such as a bio- or oosparite); packstone, grains in contact but with considerable matrix (e.g. biomicrite); wackstone, grains are floating in a matrix (could also be a biomicrite); and a mudstone, chiefly micrite with few grains (<10%). The terms can be qualified to give information on composition, e.g. oolitic grainstone, peloidal mudstone. Dolomite/dolostone Composed of > 50% of the mineral dolomite Abundant from Precambrian to Holocene Some are obviously diagenetically altered limestones Origin of fine-grained dolostones remains elusive – “dolomite problem Diagenesis After deposition, carbonate sediments are subjected to a variety of diagenetic processes – Changes in porosity, mineralogy, chemistry – Carbonate minerals more susceptible to dissolution, recrystallization, replacement than most siliciclastic minerals Carbonate minerals may experience pervasive alteration of mineralogy. E.g., aragonitecalcite, dolomitization. These changes can alter or destroy original depositional textures. Porosity may be reduced or enhanced. Depositional Texture Recognizable Original components not bound together during deposition Contains mud (particles of clay and fine silt Lacks mud and size) is grain supported Mud-supported Grainsupported Mudstone (Grains<10%) Wackstone (Grains>10%) Packstone Depositional texture not recognizable Original components were bound together during deposition as shown by intergrown skeletal matter, lamination contrary to gravity, or sedimentatfloored cavities that are roffed over by organic or questionably organic matter and are too large to be interstices. Boundstone Crystalline Carbonates (subdivided according to classifications designed to bear on physical texture or diagenesis) Grainstone (mudstone<1%) Classification of Limestone, based on depositional texture Summary Calcite, aragonite and dolomite are most common carbonate minerals. Environmental conditions need to be “just right” for deposition of carbonate sediments. These include; � Salinity, temperature, water depth, etc. � Most carbonate sediments produced biologically or by biochemical mediation Limestones consist primarily of grains (allochems), micrite and sparry calcite. Four types of carbonate grains: lithoclasts, skeletal particles, precipitates, peloids. Modified Dunham classification uses (primarily) relative proportion of grains and micrite. Dolostone (“dolomite rock”) consists of >50% dolomite. Different origins possible Diagenesis can dramatically affect mineralogy, porosity, texture of carbonate rocks. Evaporitic sediments These rocks are formed within the depositional basin from chemical substances dissolved in the seawater or lake water. Gypsum and salt are good example of evaporitic sediments. Siliceous sediments Chert is the most common chemical siliceous sediment. It is a dense rock composed of one or several forms of silica (opal, chalcedony, microcrystalline quartz). It occurs either as nodular segregations, mainly in a carbonate host rock, or as areally extensive bedded deposits. It has a tough, splintery to conchoidal fracture. It may be white or variously colored gray, green, blue, pink, red, yellow, brown, and black. Flint (feuerstein) is a term widely used both as a synonym for chert and as a variety of chert. Organic sediments Coals Coals are carbon-rich rocks that are composed of the altered remains of woody plant debris. The two principal types of coals are: 1. Lignite (brown coal): composed of loosely bound (friable) organic detritus, including some clearly recognizable plant remains 2. Bituminous coal: highly compacted black coal composed of recrystallized carbon Coal Formation • Delta, continental environments • Carbonized Woody Material • Often fossilized trees, leaves present Figure 3.16. Coal formation process. Oil shale The term oil shale has been applied to any rock from which substantial quantities of oil can be extracted by heating. Lithology is diverse and may include shales, marlstones, dolomitic limestones and siltstones. Normally these rocks are fine textured and laminated. They range in color from light shades of brown, green to dark brown, gray or black. Types of Oil Shale Oil shales can be broadly categorized in three types:1. Carbonate rich shale: those which contain abundant carbonate minerals, commonly varied, hard and tough rocks 2. Siliceous shale: those devoid of carbonate minerals with abundant siliceous minerals. They are dark brown or black colored 3. Cannel Shale: Those composed of algal remains, containing so much mineral impurities, dark brown or black color, sometimes classed as impure cannel coal, torbanite or some varieties of marine coals Volcanoclastic Sediments • • Fragmental volcanic rocks formed by any mechanism or origin emplaced in any physiographic environment (on land, under water, or under ice), or mixed with any nonvolcanic fragment types in any proportion are called Volcaniclastic sediments. Volcaniclastic materials exhibit all possible degrees of sorting. Some are very well sorted and finely laminated; others are chaotic and unsorted and contain debris ranging from the finest ash to great blocks of either cognate or noncognate (accidental) rocks. 3.3.2. Texture and Structure of Sedimentary rocks Texture Texture- refers to the size, shape, arrangement of the grains that make up the rock. • Clastic- composed of individual fragments that were transported and deposited as particles. • Crystalline- results from the in situ precipitation of solid mineral crystals. Grain size- grain diameter (boulders, pebbles, cobbles, sand, silt, or clay). Shape- is described in terms of sphericity Roundness or (angularity) refers to the sharpness or smoothness of their corners. Figure 3.17. Relationships between Sphericity and Roundness. Analyzing the parameters of clastic rocks, one may realize about the matrix content, sorting, roundness, and composition of a clastic rock at hand. An important aspect dealing with such variations is known as the maturity of the rock. Two types of maturity; textural and compositional maturities are devised to analyze degree of weathering and, energy level and persistency of transporting media, during deposition. Texturally immature sediments are those with much matrix, poor sorting and angular grains. Texturally mature sediments, on the other hand, are characterized by little matrix, moderate to good sorting and subrounded to rounded grains. Sediments with no matrix, very good sorting and well-rounded grains are known as super matured. Textural maturity in sandstones is largely a reflection of the depositional process, although it can be modified by diagenetic processes. Where there has been minimal current activity, the sediments generally are texturally immature; persistent current or wind activity results in more mature sandstone. A compositionally immature sandstone contains many unstable grains (i.e., unstable rock fragments and minerals), and much feldspar. Where rock fragments are of a more stable variety and there is some feldspar and much quartz, then the sediment is referred to as mature. For sandstone composed almost entirely of quartz grains the term supermature is aapplied. Compositional maturity can be expressed by the ratio of quartz+chert grains to feldspars+rock fragments. This compositional maturity index is useful if comparisons between different sandstones are required. Compositional maturity basically reflects the weathering processes in the source area and the degree and extent of reworking and transportation. Typically, compositionally immature sediments are located close to their source area or they have been rapidly transported and deposited with little reworking from a source area of limited physical and chemical weathering. Here a caution with the concept of compositional maturity is that they can considerably changed a) if the source area itself consists of mature sediments and b) if the sediments are supplied directly to a beach and nearshore area from adjacent igneous-metamorphic rocks. Structures The process of deposition usually imparts variations in layering, bed forms or other structures that point to the environments in which deposition occurred. Such things as water depth, current velocity and current direction can some times be determined from sedimentary structures. Thus, it is important to recognize various sedimentary structures to infer the depositional environments of ancient sediments. In the study of stratigraphy, especially in the deformed and folded areas, sedimentary structures are so important to understand which way is up/down, so that able to determine the sequence of events occurred in the area. The structural features that tell us which way is up/down are often referred to as top and bottom indicators. A. Stratification and Bedding 1. Layering (bedding): One of the most obvious features of sedimentary rocks is layering structure or stratification. The layers are evident because of differences in mineralogy, grain size, degree of sorting, or color of the different layers. In rocks, these differences may be made more prominent by the differences in resistance to weathering or color changes brought out by weathering. Layering is usually, described on the basis of layer thickness as shown in the table below. Distinctive types of layering are described below. Description of beds in accordance with their thickness Bed thicknesses (cm) 2. >300 Massive 100-300 Very thickly bedded 30-100 Thickly bedded 10-30 Medium bedded 3-10 Thinly Bedded 1-3 Very thinly bedded 0.3-1 Thickly laminated < 0.3 Thinly laminated Cross bedding: consists of sets of beds that are inclined with respect to one another. The beds are inclined in the direction that the wind or water was moving at the time of deposition. Boundaries between sets of cross beds, usually, represent an erosional surface. Cross bedding is very common in beache deposits, sand dunes, and river sediments. Individual beds within cross bedded strata are useful indicators of current direction and to indicate the top and bottom part of the bed. All cross beds have asymptotic contact in their lower contact on which they were deposited. Cross bed sets boundary Bed set Cross beds 3. Graded bedding: with decreasing of current velocity, larger or more dense particles are deposited first and followed by smaller particles. If this occurred within a bed, then it results in the formation of a bed that sows decreasing of grain size, upwards. This structure is important in determination of tops and bottoms of beds. Commonly, reverse graded bedding cannot be occurred, as current velocity increased. This is because, as the velocity of the current increased, it will start to erode the surface of the bed instead of progressive deposition of coarser materials. Upward direction of the succession Graded bed B. Surface Features These sedimentary structures are developed on the surface of the beds and tell us about water currents, wind direction and climate conditions. 1. Ripple marks: Ripple marks are characteristic of shallow water deposition. They are caused by waves or winds piling up the sediments into long ridges. Based on their geometry, two types of ripple marks are known: Symmetrical and Asymmetrical. Asymmetrical ripple marks can give an indication of current direction of water or wind direction. Symmetrical ripples formed under the condition when the water moves back and forth. Symmetrical ripple marks typify standing water with a steady back and forth movement, such as tidal action. Back and forth movement of water Schematic draw of symmetric ripple marks ( repetition of lines is to illustrate their appearance in 3D) Current or wind direction Asymmetric ripple marks (nearly vertical in the windward side, and gentle slope in the leeward side) 2. Mud cracks: These structures result from the drying out of wet sediments at the surface of the earth. The cracks are due to shrinkage of the sediments (clays), on drying. In cross section, the mud cracks tend to curl up, thus becoming a good top/bottom indicator. The presence of mud cracks indicates that the sediments were exposed to the surface shortly after deposition. - 3. Casts and Molds: Any depression formed on the surface of previously deposited sediment, at the interface of water and sediment, may become a mold for any sediment that come later. The body of the newly sediment that takes on the shape of the mold is referred to as cast. Load casts: These are bulbous protrusions that are formed when compaction causes sediment to be pushed downward in to softer sediments. Flute casts (Sole marks): Flutes are elongated depressions formed at the surface of the formerly deposited sediment by current erosion. The flutes form an elongated mold for the new sediment. Preservation of the overlying sediment as cast resulted in flute casts, which are some times referred to as sole marks. Flute casts are excellent indicators of current direction and tops/bottoms of beds. 4. Tracks and Trails: These features result from organisms moving across the sediment as they walk, crawl, or drag their body parts through the sediments. 5. Burrow marks: Any organism that burrows in to soft sediment can disturb the sediment and destroy many of the structures. If burrowing is not extensive, the holes made by such organisms can later become filled with water that deposits new sediment in the holes. Burrow marks are also used to indicate top and bottom parts of beds. If animals were churning up and intensively burrowed through the sediment, bioturbation may be resulted. Bioturbation disrupts and even destroys primary bedding and lamination. It may produce nodularity in the sediment, with subtle grain-size differences between burrow and surrounding sediments. A color mottling can be produced by burrowing organisms. 6. Slump folds: formed by down slope mass movement of sediment up on glide plane involving significant bending of sediment layers. 3.3.3. Depositional Environments of Sedimentary rocks Sediments are formed and accumulated in different places under different conditions. The sedimentary depositional environment describes the combination of physical, chemical and biological processes took place during the deposition of a particular type of sediment. Determination of depositional environment of sediments is important to construct the paleogeography and paleoclimatic condition in the study of the geologic history of an area, and to infer economically potential parts of the basin in the exploration of hydrocarbons and minerals. In most cases, ancient depositional environments are found to be analogous to the existing sedimentation areas. Important parameters to determine the depositional environment of sediment are: 1. 2. 3. 4. 5. lithologic composition and rock association texture sedimentary structures fossil content the geometry of the sediment Types of depositional environments Continental Environments - Alluvial Environment - Aeolian Environment - Fluvial Environment - Lacustrine/ lake/ Environment - Glacial Environment Transitional Environments - Deltaic Environment - Tidal Environment - Lagoonal Environment - Beach Environment Marine Environment - Shallow water marine - Deep water Marine Reef Environment Figure 3.18. Block diagram showing the types of depositional environments. 3.4. Metamorphic Rocks 3.4.1. Definitions of Metamorphism Metamorphism is defined as the mineralogical and structural adjustment of solid rocks to physical and chemical conditions that have been imposed at depths below the near surface zones of weathering and diagenesis and which differ from conditions under which the rocks in question originated. The word "Metamorphism" comes from the Greek: meta = change, morph = form, so metamorphism means to change form. In geology this refers to the changes in mineral assemblage and texture that result from subjecting a rock to conditions such pressures, temperatures, and chemical environments different from those under which the rock originally formed. Metamorphism is characterized by: (i) phase changes: - growth of new physically discrete, separable components (minerals), either with or without (isochemical) addition of new material; and/or (ii) textural changes: - recrystallization, alignment and/or grain size, usually as a result of unequal application of stress. • Note that Diagenesis is also a change in form that occurs in sedimentary rocks. In geology, however, we restrict diagenetic processes to those which occur at temperatures below 200oC and pressures below about 300 MPa (MPa stands for Mega Pascals), this is equivalent to about 3 kilobars of pressure (1kb = 100 MPa). • Metamorphism therefore occurs at temperatures and pressures higher than 200oC and 300 MPa. Rocks can be subjected to these higher temperatures and pressures as they are buried deeper in the Earth. Such burial usually takes place as a result of tectonic processes such as continental collisions or subduction. • The upper limit of metamorphism occurs at the pressure and temperature where melting of the rock in question begins. Once melting begins the process changes to an igneous process rather than a metamorphic process. Figure 3.19. Diagram showing limits of metamorphism. Factors that Control Metamorphism Metamorphism occurs because some minerals are stable only under certain conditions of pressure and temperature. When pressure and temperature change, chemical reactions occur to cause the minerals in the rock to change to an assemblage that is stable at the new pressure and temperature conditions. But, the process is complicated by such things as how the pressure is applied, the time over which the rock is subjected to the higher pressure and temperature, and whether or not there is a fluid phase present during metamorphism. Temperature Temperature increases with depth in the Earth along the Geothermal Gradient. Thus higher temperature can occur by burial of rock. Temperature can also increase due to igneous intrusion. Pressure Pressure increases with depth of burial, thus, both pressure and temperature will vary with depth in the Earth. Pressure is defined as force acting equally from all directions. It is a type of stress, called hydrostatic stress, or uniform stress. If the stress is not equal from all directions, then the stress is called a differential stress. Fluid Phase Any existing open space between mineral grains in rocks can potentially contain a fluid. This fluid is mostly H2O, but contains dissolved mineral matter. The fluid phase is important because chemical reactions that involve one solid mineral changing into another solid mineral can be greatly speeded up by having dissolved ions transported by the fluid. Within increasing pressure of metamorphism, the pore spaces in which the fluid resides is reduced, and thus the fluid is driven off. Thus, no fluid will be present when pressure and temperature decrease and, as discussed earlier, retrograde metamorphism will be inhibited. Time The chemical reactions involved in metamorphism, along with recrystallization, and growth of new minerals are extremely slow processes. Laboratory experiments suggest that the longer the time available for metamorphism, the larger are the sizes of the mineral grains produced. Thus, coarse grained metamorphic rocks involve long times of metamorphism. Experiments suggest that the time involved is millions of years. Mineral Asseemblage/Paragenesis Minerals those possessing the lowest chemical potential energy under the conditions of metamorphism are said to be in equilibrium. These equilibrium minerals referred to simply as Mineral assemblage or Mineral paragenesis. Mineral assemblages will be written as lists of mineral names separated by plus signs, thus: A+B+C Most Granoblastic texture rocks are often associated with equilibrium, but rocks without these may also have equilibrium mineral assemblages. R. Manson (1984) assumes that metamorphic rocks have equilibrium mineral assemblages unless there is definite evidence to the contrary. If a rock is to be regarded as having an equilibrium mineral assemblage are as follows: • • • • Each mineral in the assemblage list must have a boundary somewhere in the rock with all the other members. The texture must be of a type thought to have formed by metamorphic recrystallization, not by fragmentation during dynamic metamorphism or igneous crystallization from a melt. The minerals must not show compositional zoning. The minerals must not show obvious replacement textures such as reaction rims or alteration along cracks 3.4.2. Types of Metamorphism There are six types of metamorphism. These are:- 1. 2. 3. 4. 5. 6. Contact Metamorphism Regional Metamorphism Cataclastic Metamorphism Hydrothermal Metamorphism Burial Metamorphism Shock (impact) Metamorphism CONTACT METAMORPHISM Contact metamorphism is often referred to as high temperature, low pressure metamorphism. The rock produced is often a fine-grained rock that shows no foliation, called a hornfels. It occurs adjacent to igneous intrusions and results from high temperatures associated with the igneous intrusion. Since only a small area surrounding the intrusion is heated by the magma, metamorphism is restricted to the zone surrounding the intrusion, called a metamorphic or contact aureole. Outside of the contact aureole, the rocks are not affected by the intrusive event. The grade of metamorphism increases in all directions toward the intrusion. Because the temperature contrast between the surrounding rock and the intruded magma is larger at shallow levels in the crust where pressure is low, REGIONAL METAMORPHISM Occurs over large areas and generally does not show any relationship to igneous bodies. Most regional metamorphism is accompanied by deformation under non-hydrostatic or differential stress conditions. Thus, regional metamorphism usually results in forming metamorphic rocks that are strongly foliated, such as slates, schist, and gneisses. The differential stress usually results from tectonic forces that produce compressional stresses in the rocks, such as when two continental masses collide. Thus, regionally metamorphosed rocks occur in the cores of fold/thrust mountain belts or in eroded mountain ranges. Compressive stresses result in folding of rock and thickening of the crust, which tends to push rocks to deeper levels where they are subjected to higher temperatures and pressures. CATACLASTIC METAMORPHISM Occurs as a result of mechanical deformation, like when two bodies of rock slide past one another along a fault zone. Heat is generated by the friction of sliding along such a shear zone, and the rocks tend to be mechanically deformed, being crushed and pulverized, due to the shearing. Is not very common and is restricted to a narrow zone along which the shearing occurred. HYDROTHERMAL METAMORPHISM Rocks that are altered at high temperatures and moderate pressures by hydrothermal fluids. This is common in basaltic rocks that generally lack hydrous minerals. The hydrothermal metamorphism results in alteration to Mg-Fe rich hydrous minerals such as talc, chlorite, serpentine, actinolite, tremolite, zeolites, and clay minerals. Rich ore deposits are often formed as a result of hydrothermal metamorphism. BURIAL METAMORPHISM When sedimentary rocks are buried to depths of several hundred meters, temperatures greater than 300oC may develop in the absence of differential stress. New minerals grow, but the rock does not appear to be metamorphosed. The main minerals produced are often the Zeolites. Overlaps, to some extent, with diagenesis, and grades into regional metamorphism as temperature and pressure increase. SHOCK METAMORPHISM (IMPACT METAMORPHISM) When an extraterrestrial body, such as a meteorite or comet impacts with the Earth or if there is a very large volcanic explosion, ultrahigh pressures can be generated in the impacted rock. These ultrahigh pressures can produce minerals that are only stable at very high pressure, such as the SiO2 polymorphs coesite and stishovite, produce textures known as shock lamellae in mineral grains, and such textures as shatter cones in the impacted rock. 3.4.3. Grade of Metamorphism Metamorphic grade is a general term for describing the relative temperature and pressure conditions under which metamorphic rocks form. Low-grade metamorphism takes place at temperatures between about 200 to 320oC, and relatively low pressure. Low grade metamorphic rocks are generally characterized by an abundance of hydrous minerals. With increasing grade of metamorphism, the hydrous minerals begin to react with other minerals and/or break down to less hydrous minerals. High-grade metamorphism takes place at temperatures greater than 320oC and relatively high pressure. As grade of metamorphism increases, hydrous minerals become less hydrous, by losing H2O, and non-hydrous minerals become more common. As the temperature and/or pressure increases on a body of rock we say that the rock undergoes prograde metamorphism or that the grade of metamorphism increases. Whereas as temperature and pressure fall due to erosion of overlying rock or due to tectonic uplift, one might expect metamorphism to a follow a reverse path and eventually return the rocks to their original unmetamorphosed state. Such a process is referred to as retrograde metamorphism. Metamorphic Facies In general, metamorphic rocks do not undergo significant changes in chemical composition during metamorphism. The changes in mineral assemblages are due to changes in the temperature and pressure conditions of metamorphism. Thus, the mineral assemblages that are observed must be an indication of the temperature and pressure environment that the rock was subjected to. This pressure and temperature environment is referred to as metamorphic Facies. (This is similar to the concept of sedimentary facies, in that a sedimentary facies is also a set of environmental conditions present during deposition). The sequence of metamorphic facies observed in any metamorphic terrain, depends on the geothermal gradient that was present during metamorphism (Fig. 3.20). A high geothermal gradient might be present around an igneous intrusion, and would result in metamorphic rocks belonging to the hornfels facies. Under a normal geothermal gradient, would progress from zeolite facies to greenschist, amphibolite, and eclogite facies as the grade of metamorphism (or depth of burial) increased. If a low geothermal gradient was present, then rocks would progress from zeolite facies to blueschist facies to eclogite facies. Thus, if we know the facies of metamorphic rocks in the region, we can determine what the geothermal gradient must have been like at the time the metamorphism occurred. Figure 3.20. Metamorphic facies encountered during prograde metamorphism. Names of metamorphic facies and typical mineral assemblages of basic igneous rocks and pelitic rocks Facies Typical mineral assemblages in basic igneous rocks (with relict igneous plagioclase and clinopyroxene) Typical mineral assemblages in pelitic rocks not defined Zeolite smectite + zeolite (with relict igneous plagioclase) not defined Greenschist chlorite + actinolite + albite + epidote + quartz chlorite + muscovite + chloritoid + quartz Epidoteamphibolite Amphibolite hornblende + epidote albite + almandine garnet + quartz hornblende + andesine garnet + quartz clinopyroxene + labradorite + orthopyroxene + quartz Prehnitepumpellyite Granulite Medium pressure and Medium almandine garnet + chlorite + temperature muscovite+ biotite + quartz garnet + biotite + muscovite + sillimanite + quartz garnet + cordierite + biotite + sillimanite + quartz Pyroxene hornfels Sanidinite clinopyroxene + labradorite + quartz clinopyroxene + labradorite + Quartz Glaucophane schist glaucophane + lawsonite + quartz Eclogite pyrope-garnet + omphacite and clinopyroxene) cordierite + andalusite + biotite + quartz sanidine + sillimanite + hypersthene + cordierite + quartz muscovite + chlorite + spessartine garnet + quartz not known Low pressure and High temperature High pressure and Low temperature Metamorphism and Plate Tectonics At present, the geothermal gradients observed are strongly affected by plate tectonics. Along zones where subduction is occurring, magmas are generated near the subduction zone and intrude into shallow levels of the crust. Because high temperature is brought near the surface, the geothermal gradient (region A in Fig. 3.21), in these regions becomes high and contact metamorphism (hornfels facies) results. Because compression occurs along a subduction margin (the oceanic crust moves toward the volcanic arc) rocks may be pushed down to depths along either a normal or slightly higher than normal geothermal gradient. Actually the geothermal gradient is likely to be slightly higher, because the passage of magma through the crust will tend to heat the crust somewhat. In these regions (region B in Fig. 3.21), we expect to see greenschist, amphibolite, and granulite facies metamorphic rocks. Along a subduction zone, relatively cool oceanic lithosphere is pushed down to great depths. This results in producing a low geothermal gradient (temperature increases slowly with depth). This low geothermal gradient results in metamorphism into the blueschist and eclogite facies (region C in Fig. 3.21). Figure 3.21. Relationships between metamorphism and plate tectonics. 3.4.4. Classification of Metamorphic rocks Classification of metamorphic rocks is based on mineral assemblage, texture, protolith, and bulk chemical composition of the rock. Metamorphic rock names are commonly derived utilizing any one, or a combination of the following criterion (Yardley, 1989): 1) The nature of the parent material (bulk composition) 2) The rock's texture (grain size and fabric development) 3) The metamorphic mineralogy 4) Any appropriate special name Classification of metamorphic rocks based on the nature of the parent material (bulk composition) Parent Material Rock type Argillaceous/clay-rich sediments (lutites) Pelites Arenaceous (predominately sand-size) sediments Clay-sand mixtures Quartz-sand (quartz arenite) Psammites Semi-pelite Quartzite Marl (lime muds) Limestone or dolostone Volcanics (basalt, andsite, rhyolite, etc.) Ultramafics Calc-silicate/calcareous Marble Metavolcanics (metabasite (metandesite….etc.) Metaultramafics Pelitic. These rocks are derivatives of aluminous sedimentary rocks like shales and mudrocks. Because of their high concentrations of alumina they are recognized by an abundance of aluminous minerals, like clay minerals, micas, kyanite, sillimanite, andalusite, and garnet. Quartzo-Feldspathic. Rocks that originally contained mostly quartz and feldspar like granitic rocks and arkosic sandstones will also contain an abundance of quartz and feldspar as metamorphic rocks, since these minerals are stable over a wide range of temperature and pressure. Those that exhibit mostly quartz and feldspar with only minor amounts of aluminous minerals are termed quartzofeldspathic. Calcareous. Calcareous rocks are calcium rich. They are usually derivatives of carbonate rocks, although they contain other minerals that result from reaction of the carbonates with associated siliceous detrital minerals that were present in the rock. At low grades of metamorphism calcareous rocks are recognized by their abundance of carbonate minerals like calcite and dolomite. With increasing grade of metamorphism these are replaced by minerals like brucite, phlogopite (Mg-rich biotite), chlorite, and tremolite. At even higher grades anhydrous minerals like diopside, forsterite, wollastonite, grossularite, and calcic plagioclase. Basic. Just like in igneous rocks, the general term basic refers to low silica content. Basic metamorphic rocks are generally derivatives of basic igneous rocks like basalts and gabbros. They have an abundance of Fe-Mg minerals like biotite, chlorite, and hornblende, as well as calcic minerals like plagioclase and epidote. Magnesian. Rocks that are rich in Mg with relatively less Fe, are termed magnesian. Such rocks would contain Mg-rich minerals like serpentine, brucite, talc, dolomite, and tremolite. In general, such rocks usually have an ultrabasic protolith, like peridotite, dunite, or pyroxenite. Ferruginous. Rocks that are rich in Fe with little Mg are termed ferriginous. Such rocks could be derivatives of Fe-rich cherts or ironstones. They are characterized by an abundance of Fe-rich minerals like greenalite (Fe-rich serpentine), minnesotaite (Fe-rich talc), ferroactinolite, ferrocummingtonite, hematite, and magnetite at low grades, and ferrosilite, fayalite, ferrohedenbergite, and almandine garnet at higher grades. Manganiferrous. Rocks that are characterized by the presence of Mn-rich minerals are termed manganiferrous. They are characterized by such minerals as Stilpnomelane and spessartine. Textural classification The textures are used to differentiate the relative timing of crystal growth and deformation. Like any field, there is a large terminology developed. Below is a list of common textures. Note: Many of the terms for metamorphic textures contain the suffix "blastic ". Terms related to crystals: shape, orientation, and content: Porphyroblast: a mineral that is larger than its neighbors which grew in the solid state. Porphyroblast – Idioblast: euhedral (well developed crystal faces) porphyroblast – Xenoblast: anhedral (poorly developed crystal faces) porphyroblast Granoblastic polygonal: a texture in which all grains are about the same size and have planar boundaries intersecting at approximately 120 degrees. Poikiloblastic: a texture produced when a growing crystal face has enveloped inclusions from its surroundings. Poikiloblastic Corona: a ring of one or more minerals around another mineral or structure, formed by reaction with its surroundings. Pseudomorph: produced when one or more minerals replaces another mineral while retaining its crystal shape. Terms related to deformation and timing of recrystallization: - Relict: A texture of mineral that is inherited from unmetamorphosed rock or from another metamorphic grade (e.g., bedding). - Helicitic: applies to porphyroblasts or porphyroclasts possessing internal foliations (Si) that are curved. Metamorphic Fabric Mineralogical classification The most distinguishing minerals are used as a prefix to a textural term. Thus, a schist containing biotite, garnet, quartz, and feldspar, would be called biotite-garnet schist. A gneiss containing hornblende, pyroxene, quartz, and feldspar would be called hornblende-pyroxene gneiss. A schist containing porphyroblasts of garnet would be called garnet porphyroblastic schist. If a rock has undergone only slight metamorphism such that its original texture can still be observed then the rock is given a name based on its original name, with the prefix meta- applied. For example: metabasalt, metagraywacke, meta-andesite, metagranite. Special metamorphic rocks Amphibolites: These are medium to coarse grained, dark colored rocks whose principal minerals are hornblende and plagioclase. They result from metamorphism of basic igneous rocks. Foliation is highly variable, but when present the term schist can be appended to the name (i.e. amphibolite schist). Marbles: These are rocks composed mostly of calcite, and less commonly of dolomite. They result from metamorphism of limestones and dolostones. Some foliation may be present if the marble contains micas. Eclogites: These are medium to coarse grained consisting mostly of garnet and green clinopyroxene called omphacite, that result from high grade metamorphism of basic igneous rocks. Eclogites usually do not show foliation. Quartzites: Quartz arenites and chert both are composed mostly of SiO2. Since quartz is stable over a wide range of pressures and temperatures, metamorphism of quartz arenites and cherts will result only in the recrystallization of quartz forming a hard rock with interlocking crystals of quartz. Such a rock is called a quartzite. Serpentinites: Serpentinites are rocks that consist mostly of serpentine. These form by hydrothermal metamorphism of ultrabasic igneous rocks. Soapstones: Soapstones are rocks that contain an abundance of talc, which gives the rock a greasy feel, similar to that of soap. Talc is an Mg-rich mineral, and thus soapstones from ultrabasic igneous protoliths, like peridotites, dunites, and pyroxenites, usually by hydrothermal alteration. Skarns: Skarns are rocks that originate from contact metamorphism of limestones or dolostones, and show evidence of having exchanged constituents with the intruding magma. Thus, skarns are generally composed of minerals like calcite and dolomite, from the original carbonate rock, but contain abundant calcium and magnesium silicate minerals like andradite, grossularite, epidote, vesuvianite, diopside, and wollastonite that form by reaction of the original carbonate minerals with silica from the magma. The chemical exchange is that takes place is called “Metasomatism”. Mylonites: Mylonites are cataclastic metamorphic rocks that are produced along shear zones deep in the crust. They are usually fine-grained, sometimes glassy, that are streaky or layered, with the layers and streaks having been drawn out by ductile shear. Migmatites: a mixed rock of schistose or gneissic portion intimately mixed with veins of apparently quartzo-feldspathic material (known as leucosomes). Migmatites and its related terms are best reserved for regional field studies and should not be used in hand specimen descriptions. 3.4.5. Structure of Metamorphic rocks If differential stress is present during metamorphism, it can have a profound effect on the texture of the rock. Rounded grains can become flattened in the direction of maximum compressional stress. Minerals that crystallize or grow in the differential stress field may develop a preferred orientation. Sheet silicates and minerals that have an elongated habit will grow with their sheets or direction of elongation orientated perpendicular to the direction of maximum stress. This is because growth of such minerals is easier along directions parallel to sheets or along the direction of elongation and thus will grow along 3 or 2, perpendicular to 1. The type of structures formed during metamorphism is represented as follows: Slates/phyllites form at low metamorphic grade by the growth of fine grained chlorite and clay minerals. The preferred orientation of these sheet silicates causes the rock to easily break planes parallel to the sheet silicates, causing a slatey cleavage. Schist - The size of the mineral grains tends to enlarge with increasing grade of metamorphism. Eventually the rock develops a near planar foliation caused by the preferred orientation of sheet silicates (mainly biotite and muscovite). Quartz and feldspar grains however show no preferred orientation. The irregular planar foliation at this stage is called schistosity Gneiss As metamorphic grade increases, the sheet silicates become unstable and dark colored minerals like hornblende and pyroxene start to grow. These dark colored minerals tend to become segregated into distinct bands through the rock (this process is called metamorphic differentiation), giving the rock a gneissic banding. Because the dark colored minerals tend to form elongated crystals, rather than sheet- like crystals, they still have a preferred orientation with their long directions perpendicular to the maximum differential stress. Granulite - At the highest grades of metamorphism most of the hydrous minerals and sheet silicates become unstable and thus there are few minerals present that would show a preferred orientation. The resulting rock will have a granulitic texture that is similar to a phaneritic texture in igneous rocks. In general, the grain size of metamorphic rocks tends to increase with increasing grade of metamorphism, as seen in the progression form fine grained shales to coarser (but still fine) grained slates, to coarser grained schists and gneisses. Figure 3.22. Structural development in metamorphic rocks.