Supplementary Materials L14-06564 (Sep 11, 2014).
advertisement

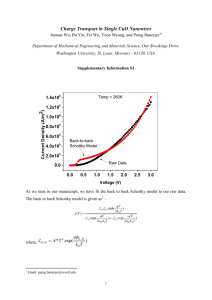
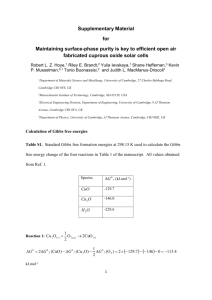
Supplementary Materials for: Hybrid CuO/SnO2 nanocomposites: towards cost-effective and high performance binder free lithium ion batteries anode materials G. Z. Xing,1,2 Y. Wang,1 J. I. Wong,1 Y. M. Shi,1 Z. X. Huang,1 S. Li2 and H. Y. Yang1,a) 1 Pillar of Engineering Product Development, Singapore University of Technology and Design, 20 Dover Drive, 138682, Singapore 2 School of Materials Science and Engineering, The University of New South Wales, Sydney, New South Wales 2052, Australia a) Email: yanghuiying@sutd.edu.sg Synthesis of Nanocomposites: Briefly, CuO nanostructures on Cu foil (CuO/Cu) were synthesized first by heating 10 µm thick Cu foil plates by a hotplate at 300 oC for 16 hrs at atmosphere. After that, 10 ml 4.22×10-4 mol/ml Sn(CH3CO2)2 dissolved in ethanol was carefully dropped onto 10 pieces of CuO/Cu foils and baked on a hotplate at 300 oC for 20 mins until all the samples were dried thoroughly with white colour appearance. Then the prepared products were transferred into a tube furnace and annealed at 500 oC for 1 h with protection gas of Ar (flowing rate: 200 sccm). The SnO2/Cu control sample was synthesized by the same procedure without CuO synthesis step. It is critical to correctly determine the weights of the active layer materials in the performance evaluation of LIBs. In our experiments, since the Cu in CuO nanostructures comes from the Cu foil substrate, to extract how much exactly of CuO formed from the oxidation of Cu during the heating process, totally 30 blank Cu foils were weighted before beating and the final products were balanced to deduct the original total weight for an averaged determination of total oxygen mass on each sample. Consequently, the eventual weight of CuO on individual Cu foil was calculated according to the atomic mass ratio between O and Cu. Accordingly, the weight of the active CuO was about 0.75 mg per piece. The backside of CuO layer was removed to expose the bared Cu foil for good conductivity. In order to prevent the active material peel off from the Cu foil current collector, the active material with Cu foil was pressed with the nickel foam. Characterizations: The morphologies and detailed crystal structures of samples were investigated by field-emission scanning electron microscopy (FESEM, JSM-7600) and transmission electron microscopy (TEM, JEM2100F), respectively. Structural characteristics of the nanocomposite samples were measured by X-ray diffraction (XRD, Siemens, D5005) with Cu Ka (λ=0.154 nm) radiation under the accelerating voltage of 40 kV. Raman spectra were performed by a confocal Raman system with the laser excitation at 532 nm (WITec Instruments Corp, Germany). Electrochemical Measurements: The electrochemical performance of CuO, SnO2 and CuO/SnO2 nanocomposites on Cu foils were measured by assembling into a typical two-electrode half-cell configuration, composed of the active material as the working electrode, a lithium foil as the counter electrode, Celgard 2400 membrane as the separator between the working and counter electrodes, and 1 M LiPF6 solution dissolved in a mixture of ethylene-carbonate–dimethyl-carbonate (EC–DMC, 1:1 in volume) as the electrolyte. The cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) measurements were performed by an electrochemical workstation (VMP3, Bio-logic, France). The galvanostatic charge/discharge test was carried out in the voltage range of 0.01-3 V vs. Li/Li+ at various current densities ranging from 100 to 2000 mA g-1 by a battery analyzer (Neware, Shenzhen). Figure S1. (a) EDX spectrum of the CuO/SnO2 nanocomposites suspend on C lacey film supported on TEM Cu grid, and the corresponding elements mapping with clear distribution of (b) C, (c) Cu, (d) O and (d) Sn. Figure S2. CV curves of (a) CuO and (b) SnO2 nanocomposites electrodes of the first three cycles at a scan rate of 0.1 mV s-1 in a potential range of 0.01–3 V vs. Li/Li+. Galvanostatic discharge/charge curves of (c) CuO and (d) SnO2 nanocomposites electrodes at a current density of 500 mA g-1 for the first 5 cycles. Meaning of the symbols in Fig. 4 and Table 1 Rs represents the electrolyte resistance corresponding to the intercept of the semicircle at high frequency range; Rf and Q1 are the SEI layer resistance and the constant phase element (CPE), respectively, corresponding to the semicircle at high frequency range; Rct and Q2 are the charge transfer resistance and related double layer capacitor, respectively, corresponding to the semicircle in high-middle frequency region; and W is Warburg impedance corresponding to the straight line in low frequency range related to the lithium-diffusion process.1 Reference 1. S.-Y. Liu, J. Xie, Y.-X. Zheng, et al., Electrochim. Acta 66, 271 (2012).