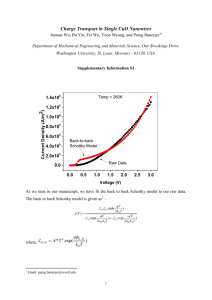
Cu2O rlzh - supplementary material
advertisement

Supplementary Material for Maintaining surface-phase purity is key to efficient open air fabricated cuprous oxide solar cells Robert L. Z. Hoye,1 Riley E. Brandt,2 Yulia Ievskaya,1 Shane Heffernan,3 Kevin P. Musselman,4,1 Tonio Buonassisi,2 and Judith L. MacManus-Driscoll1 1Department of Materials Science and Metallurgy, University of Cambridge, 27 Charles Babbage Road, Cambridge CB3 0FS, UK 2 Massachusetts Institute of Technology, Cambridge, MA 02139, USA 3Electrical Engineering Division, Department of Engineering, University of Cambridge, 9 JJ Thomson Avenue, Cambridge CB3 0FA, UK 4 Department of Physics, University of Cambridge, JJ Thomson Avenue, Cambridge, CB3 0HE, UK Calculation of Gibbs free energies Table S1. Standard Gibbs free formation energies at 298.15 K used to calculate the Gibbs free energy change of the four reactions in Table 1 of the manuscript. All values obtained from Ref. 1. Reaction 1: Cu 2 O ( s ) Species G 0 f (kJ.mol-1) CuO -129.7 Cu2O -146.0 H 2O -228.6 1 O 2 2CuO ( s ) 2 (g) 1 G 0 2G 0 f (CuO ) G 0 f (Cu 2 O) G 0 f (O 2 ) 2 129.7 146 0 113.4 2 kJ.mol-1 1 Reaction 2: 2CuO ( s ) H 2 ( g ) Cu 2 O( s ) H 2 O( g ) G 0 G 0 f (Cu 2 O) G 0 f (H 2 O) 2G 0 f (CuO) G 0 f (H 2 ) G 0 146 228.6 2 129.7 0 115.0 kJ.mol-1 Reaction 3: Cu 2 O( s ) H 2 ( g ) 2Cu ( s ) H 2 O( g ) G 0 2G 0 f (Cu) G 0 f (H 2 O) G 0 f (Cu 2 O) G 0 f (H 2 ) G 0 0 228.6 146 82.6 kJ.mol-1 Reaction 4: CuO ( s ) H 2 ( g ) Cu ( s ) H 2 O( g ) G 0 G 0 f (Cu) G 0 f (H 2 O) G 0 f (CuO) G 0 f (H 2 ) G 0 0 228.6 129.7 0 98.9 kJ.mol-1 Thermodynamic considerations: One way of controlling whether Cu, Cu2O or CuO is the thermodynamically stable phase at a certain temperature is to adjust the partial pressure of oxygen in the gas mixture over the compound. This oxygen partial pressure can be controlled by adjusting the gas ratio in a H2/CO2 mixture. From the performance phase diagram of the Cu-O system,2 Cu2O is thermodynamically stable at an oxygen partial pressure between 1 × 10-38 Pa and 1 × 10-28 Pa at 100 °C, which is approximately the AP-SALD (atmospheric pressure spatial atomic layer deposition) deposition temperature that would be used.3,4 The oxygen partial pressure cannot be controlled to such a low level, and these equilibrium calculations also indicate that Cu2O should form CuO in open air. It can be seen from Figure 1 in the manuscript that this does happen, but that the extent of this reaction is time-dependent. This indicates that kinetic (rather than thermodynamic) considerations are more important over the temperature range we are interested in. 2 Kinetic considerations: The order and parameters influencing the rates of Reactions 1 – 4 in the manuscript need to be experimentally determined. But to illustrate the importance of controlling the partial pressure of H2 in the gas mixture ( p H 2 ) depending on the partial pressure of O2 ( p O 2 ) in the gas mixture under the APSALD gas manifold, we here consider the simplest case where the reactions are independent, first order and linear. For Reaction 1, the reaction rate ( r1 ) is given by Eq. S1: r1 2k1 (T ) pO 2 (S1) where k1 (T ) is the rate constant for Reaction 1 and p O 2 the oxygen partial pressure in the system. For Reaction 2, the reaction rate ( r2 ) is given by Eq. S2: r2 k 2 (T ) p H 2 (S2) where k 2 (T ) is the rate constant for Reaction 2 and p H 2 the hydrogen partial pressure in the system. To prevent the formation of CuO and reduce any CuO surface layers present, r2 r1 k 2 (T ) p H 2 2k1 (T ) pO2 pH2 (S3) 2k1 (T ) p O2 k 2 (T ) (S4) Eq. S4 shows that if the size of the inert gas channels under the AP-SALD gas manifold is reduced (see Ref. 5) or if the inert gas supply has a higher concentration of O2, then the minimum p H 2 needed to prevent CuO formation will need to be controllably increased to offset the increased p O 2 . 3 References 1 L.I. Berger et al., in Handbook of Chemical Physics, 95th ed., edited by W.M. Haynes (CRC Press, Boca Raton, FL, 2014), Chap. 5, pp. 4 - 42. 2 A.D. Pelton, in Physical Metallurgy Vol. I, edited by D. Laughlin and K. Hono (Elsevier, Amsterdam, 2014), Chap. 3, pp. 203 - 303. 3 Y. Ievskaya, R.L.Z. Hoye, A. Sadhanala, K.P. Musselman, and J.L. MacManus-Driscoll, Sol. Energy Mater. Sol. Cells In Press (2014). http://dx.doi.org/10.1016/j.solmat.2014.09.018 4 R.L.Z. Hoye, S. Heffernan, Y. Ievskaya, A. Sadhanala, A.J. Flewitt, R.H. Friend, J.L. MacManus-Driscoll, and K.P. Musselman, ACS Appl. Mater. Interfaces 6, 22192 (2014). http://dx.doi.org/10.1021/am5058663 5 D. Muñoz-Rojas and J. MacManus-Driscoll, Mater. Horiz. 1, 314 (2014). http://dx.doi.org/10.1039/C3MH00136A 4