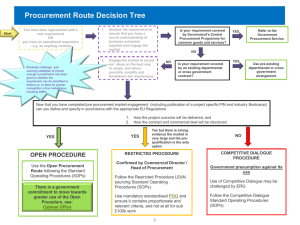
GOSH PPBP v1.1 - NHS London Procurement Partnership
advertisement

Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation Electronic Patient Records and Clinical / Business Intelligence and Research Platform-enabled Transformation Programme Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation Version 1.1 Version: 1.1 Date: Commercially Confidential Version 1.1 23rd December 2015 Page 1 of 72 Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation Change History Version Reason/Summary of Changes Date 1.0 First issue 1.1 Second issue, inserted OMNI-Lab at p69 23/12/2015 GOSH Commercially Confidential Version 1.1 18/12/15 Author GOSH/High Resolution Consulting Page 2 of 72 Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation Distribution List for Review and Approval Name Commercially Confidential Version 1.1 Title Version and Date Page 3 of 72 Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation TABLE OF CONTENTS 1. PURPOSE OF THIS DOCUMENT ............................................................................... 5 2. INTRODUCTION AND BACKGROUND TO THE TRUST ................................................ 7 3. A UNIQUE OPPORTUNITY ....................................................................................... 9 4. THE STRATEGIC CASE ............................................................................................ 12 5. EPR REQUIREMENTS............................................................................................. 15 6. CLINICAL / BUSINESS INTELLIGENCE AND RESEARCH PLATFORM REQUIREMENTS . 24 7. IMPLICATIONS OF A LOT BASED PROCUREMENT ................................................... 35 8. BUDGET, CONTRACT AND POTENTIAL PROVIDER ISSUES ....................................... 37 9. CONDUCT OF THE PROCUREMENT ........................................................................ 45 10. POTENTIAL PROVIDER BRIEFING EVENT ................................................................ 58 11. APPENDICES ......................................................................................................... 61 Commercially Confidential Version 1.1 Page 4 of 72 Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation 1. PURPOSE OF THIS DOCUMENT 1.1 INTRODUCTION This Potential Provider Briefing Paper provides essential background information and context to the Trust and to this Electronic Patient Record (EPR)-enabled transformation procurement. In the first instance, it invites Potential Providers to respond to a range of issues designed to inform the Trust’s thinking and the Trust’s design of the procurement. In the second instance, it is intended to inform pre-procurement market engagement and Potential Provider decision making as to whether to bid for this exciting and unique opportunity. In the third instance, an updated version of the paper, that reflects the outcomes of that pre-procurement market engagement will be issued with the PQQ documentation and will provide essential background information and context that Potential Providers should refer to, and draw on, in framing their PQQ responses. 1.2 POTENTIAL PROVIDER INVITATION Potential Providers are invited to: Respond to the Trust’s request for indicative pricing for a world-class EPR (see Appendix 1) Book a one to one meeting to discuss EPR requirements with the Trust on the afternoon of 21st January 2015 (see Appendix 2) Book a one to one meeting to discuss Clinical / Business Intelligence and Research Platform requirements with the Trust on 22nd January 2015 (see Appendix 3) Raise any clarification questions with the Trust via its procurement portal at https://www.lppsourcing.org/procontract/lpp/supplier.nsf/. These questions will be incorporated within the FAQ document discussed at Section 10.4 (and to be issued with the OJEU Contract Notice) Please note that, reflecting its unique, paediatric-related requirements, the Trust will limit the availability of discussions to those Potential Providers able to conclusively demonstrate directly relevant, proven, world class solutions that have been successfully delivered in healthcare organisations of a similar size and nature to the Trust. Leading on from the above, and for the avoidance of doubt, please note that the Trust is clear that: It does not wish to enter into a development partnership for core EPR requirements, including PAS requirements It prefers to contract with a Clinical/Business Intelligence and Research Platform Potential Provider that possesses significant healthcare experience and expertise Appendices 2 and 3 provide the Trust’s proformas to be used to request a one to one meeting with the Trust. Slots will be allocated on a first come, first served, basis. Commercially Confidential Version 1.1 Page 5 of 72 Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation 1.3 THE TRUST’S LOT BASED APPROACH TO THE PROCUREMENT Subject to the outcome of the Trust’s pre-procurement market engagement, the following lots will form the basis of the procurement: Lot 1 - An EPR solution Lot 2 - A Clinical / Business Intelligence and Research Platform Commercially Confidential Version 1.1 Page 6 of 72 Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation 2. INTRODUCTION AND BACKGROUND TO THE TRUST The vision of Great Ormond Street Hospital for Children NHS Foundation Trust (GOSH) is to be the leading children’s hospital in the world. It aims to hold that position through the quality of its care, the service it provides, the efficiency it delivers and its research outcomes. Many of the children and young people we see have been referred to GOSH because they need specialist care that their local hospital cannot provide. We have a strong brand which is recognised and trusted. Our employees have the skills to provide a safe, effective and world-class service. The hospital’s mission and commitment is to put children at the heart of everything we do, ‘the child first and always’. We call them Our Always Values. So we will: Always be Welcoming Always be Helpful Always be Expert Always be One Team Following our Listening Event in 2013 we identified areas which our patients and their families told us needed improvement: long waits; poor communication; appointments running late. Any investment in a digital hospital must address these concerns directly. We must make sure that any new technology that we bring into the hospital can be accessed by our patients and their families and used to enhance our relationship with them. We know that many of our staff are frustrated by the current IT systems, which are complex and often difficult to use. The information is not readily transferred between systems. Paper is still a large part of the process. Core systems in use at the Trust are detailed in Appendix 4. We also know that there are real issues for patient safety with multiple ways both electronic and manual to prescribe drugs and clinical information held in separate computer systems. Clinicians tell us how they have to log-on to many different systems to piece together the whole picture and that there is always a risk that critical information will be missed. The digital hospital will include clinical decision support – giving clinicians access to pertinent information on best practice and prompting them to follow clinical guidelines, including complex treatment and prescribing pathways. This will give us the ability to audit and demonstrate our adherence to best practice with the consequent improvements in patient safety and clinical quality as well as financial performance. The digital hospital will also support the research community, using systems to identify patients who could qualify for clinical trials whilst maintaining the stringent ethical and information governance standards associated with such activity. Access to a clinical database for audit and research would remove the need for local departmental databases. This doesn’t necessarily mean a single computer system and GOSH wishes to consider the benefits, risks and costs of the various options available to us. Commercially Confidential Version 1.1 Page 7 of 72 Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation Mobile and wireless technology must be supported and a variety of user-friendly input devices: touch screens; tablets; iPads; voice recognition; barcodes and radiofrequency ID systems – we intend to provide our staff with a multiplicity of access points and devices to a single patient record. Technology on its own will not deliver these outcomes. Any investment will need to be supported by cultural change across the whole organisation. It will see some roles disappear and other roles change, and will require our staff to undertake training and gain new computer skills. Our clinicians will see changes to the way they work in clinics, theatres and on the wards. Our digital hospital vision is that every member of the team caring for a child can always access the relevant information that they need rapidly and from a single place. It is also that patients, parents and carers in other hospitals and care settings can see relevant records and contribute information in-between visits to Great Ormond Street Hospital. Commercially Confidential Version 1.1 Page 8 of 72 Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation 3. A UNIQUE OPPORTUNITY 3.1 INTRODUCTION The Trust firmly believes that this procurement offers unique opportunities for the ‘right’ Potential Provide to: 3.2 Develop a true, clinically driven, joint-working based partnership with an innovative, world class and forward looking Trust over the next 10 years or more Deliver a world-class clinical quality improvement programme via the use of leading edge paediatric-focussed technology Establish, in partnership with the Trust, the leading paediatric-focussed research facility in the world through the use of innovative healthcare informatics NATURE OF THE RELATIONSHIP AND PARTNERSHIP PRINCIPLES The Trust will expect a Potential Provider wanting to work with them on this exciting transformation programme to describe how they intend to ensure that our patients and staff gain from successful delivery. Subject to excellent Potential Provider performance, the Trust would welcome a close relationship for many years to come. A partnership based relationship involving flexible working, innovation, benefits realisation, flexible forms of funding, shared developments and shared research is open for discussion. The foundation of such a relationship must be the highly successful initial implementation of core EPR requirements, the delivery of an impressive benefits package and highly effective functionality to satisfy optional and future requirements. For the avoidance of doubt: The chosen partner must undertake to work very closely, and to establish a genuine, clinically orientated joint-working partnership with, the Trust The Trust is looking to develop and maintain genuine partnership working, in terms of shared programme management and encompassing all aspects of agreeing and planning solution implementation, workflow transition and benefits realisation The Trust expects that the chosen Potential Provider will meet the agreed contractual commitments as a key part of demonstrating its commitment to the partnership Thereafter, the Trust envisages that the genuine joint working referred to above would lead to the implementation of agreed development initiatives over time in which financial benefit and risk would be shared The Trust recognises that Potential Providers who might be interested in bidding for this exciting opportunity might not themselves possess all the necessary capacity and capability to undertake all of the transformation and benefits related activity needed. The Trust is content to receive PQQ responses, Initial Tenders and Final Commercially Confidential Version 1.1 Page 9 of 72 Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation Tenders that reflect a well-established prime EPR Solution Provider and/or subcontracted solution application and/or sub-contracted human resource partner arrangement 3.3 PARTNERSHIP WORKING The Trust wishes to emphasise its commitment to act not only as a reference site, but also as a development site and sounding board for Potential Provider developments, new modules etc. with the right partner, particularly in the areas of the child-friendly, ageappropriate patient portal, the paediatric-specific elements of the EPR and the Clinical/ Business Intelligence and Research Platform. For example, the Trust is involved in a Joint Venture with Congenica Ltd for the genomic database development and has partnered with smaller ICT suppliers for the development of departmental systems (for example dietetics and systems supporting rare disease conditions). Leading on from the above, the Trust’s initial thinking on how the partnership will manifest itself, and subject to discussion with shortlisted Potential Providers during the PreProcurement market engagement, Initial Tender and Dialogue Stages of the procurement, is summarised below: 3.4 The Potential Provider will have a key stake in the delivery of the Trust’s clinical digital strategy The establishment of a Partnership Board that: o Has joint membership between the Trust and its digital solution Potential Providers o Oversees the delivery of the contracted implementation and related transformation and benefits realisation activities o Promotes, generates, and makes decisions on further opportunities The Trust will act not only as an implementation reference site, but also as a development site and sounding board for Potential Provider developments, new modules etc., particularly in paediatric care The Potential Provider will support the Trust in exploiting new opportunities or in responding to new nationally-led initiatives DEVELOPING THE PARTNERSHIP DURING THE PROCUREMENT As stated, the Trust will adopt an open and consultative approach with those Potential Providers to be shortlisted to receive the Invitation to Participate in Dialogue (ITPD) and to participate in the Dialogue Stage of the procurement. In particular: The Invitation to Submit initial Tenders (ISIT) documentation will provide further Trust thinking surrounding all of the above and invite Potential Provider suggestions and recommendations on the most effective means of developing a true partnership that meets the respective needs of the Trust and of the successful Potential Provider Commercially Confidential Version 1.1 Page 10 of 72 Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation The Trust’s feedback on those Tenders will be contained within the Potential Provider specific ITPD documentation and will provide a focus for Dialogue Sessions that will require Potential Providers to build upon those Tenders and to develop increasingly mature proposals for the Trust to consider. That feedback will be designed to: o Ensure there is shared agreement on the proposed nature of the partnership o Develop a shared approach to the definition of commercial principles to reflect a partnership o Agree how these shared agreements and approaches should be reflected in Final Tenders and in the associated draft contract documentation to be produced in response to the Invitation to Submit Final Tender (ISFT) Commercially Confidential Version 1.1 Page 11 of 72 Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation 4. THE STRATEGIC CASE This section looks at what the hospital’s main priorities are for the future, and how technology can best fit and support these aims and aspirations. 4.1 CLINICAL SYSTEMS STRATEGY The EPR should form part of a wider overall IT strategy for the organisation. This wider IT strategy will be developed over the course of 2016/17 and should be in place before the final investment approval is made. The selection of EPR and research platform hosting will be resolved as part of that wider strategy. The three main benefits of our new electronic patient record system will be: Driving up the quality of care and enhancing patient safety The systems will support and enable clinical decision making. At the touch of a button clinical teams will be able to access all the information about a patient that they need to inform decision making. It will also give teams prompts to follow best practice guidelines, including complex treatment and prescribing pathways. The system will also support improved safety by taking multiple systems that prescribe drugs and capture clinical information which together, in one place, will minimise the risk that critical information will be missed. Patients and their families become partners and the patient experience is enhanced The system will improve the day to day administration of patient care, to make it more efficient so that it does not distract from, and even allows more time to, providing excellent clinical care and patient experience. Portals on the system will allow patients, their families and caregivers in other organisations to access appropriate clinical information to enhance joint decision making and improve the experience for the child and family. We will be able to take the child’s record to the bedside using mobile devices, allowing them to see what’s on the computer, understand what we are recording about them and why. They will be confident that we have reliable records about them. The Trust is anticipating that bespoke development will be required in order to deliver the required functionality of the portal. Supporting research breakthroughs The systems will identify patients who could qualify for trials and provide a rich source of data for audit and research of national and international significance, to underpin continuous improvement and research-led developments in medical care. The motto of the hospital is “the child first and always” and our aim is to make sure our clinical outcomes keep us among the world’s top five children’s hospitals and enable us to provide evidence of our primacy in that peer group. Commercially Confidential Version 1.1 Page 12 of 72 Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation Clinical research is of strategic importance and we are, and wish to remain, one of the top five Paediatric Research institutions in the world measured by research outcomes. Our strategy is to develop measures and improve standards so that we can provide evidence of being the top paediatric institution in the world. 4.2 CURRENT INFORMATION TECHNOLOGY PROVISION & STRATEGY 4.2.1 Infrastructure and ICT services To date GOSH has invested in core hardware and technology infrastructure with new networks, wireless access, mobile technology and upgraded desktop PCs. Video conferencing, satellite and HD video recording across 10 theatres has put GOSH at the cutting edge integrated communication. We recognise that this investment will need to be maintained and enhanced to support our digital hospital vision and seek to understand this further as part of this pre-procurement market engagement exercise. ICT Services are currently provided in-house except for network support and some service desk provision which is outsourced. The Trust will be considering its approach to infrastructure support and ICT service provision and this will be influenced by the implementation of EPR but this procurement does NOT include neither ICT infrastructure support nor EPR hosting, both of which will be considered separately. 4.2.2 Data Warehouse The Trust currently has a SQL Server and QlikView data warehouse environment which is used for statutory reporting and based on the current i.PM PAS system and several other systems. This will need to be replaced or updated alongside the EPR programme as part of the Business Intelligence solution specified as part of the Clinical / Business Intelligence and Research Platform. 4.2.3 Electronic Document and Records Management The Trust is currently digitising its historical paper records and rolling out the Kainos Electronic Document and Records Management (EDRM) system. This will remain in place for a period of time after implementation of EPR and will need to be integrated with it. This system has some eForms functionality which will be replaced by EPR. Eventually the Trust would consider the move of the data in EDRM into the Vendor Neutral Archive (VNA) (see below). 4.2.4 Image Stores The Trust has a GE PACS and image archive for Radiology and many separate image capture and storage systems for other specialties, for example, Cardiology, medical illustration, ophthalmology, etc., that we seek to integrate. The Digital Information Technology Strategy has been agreed and decisions on the date and procurement route for changing the PACS are being considered. This procurement does NOT include a PACS, but it does include the Commercially Confidential Version 1.1 Page 13 of 72 Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation option for the EPR supplier to provide or be prime contractor for a VNA for images, videos, and documents in the wide variety of forms that are available as standards. In order to facilitate the presentation and use of these artefacts as part of an integrated patient record the Trust will in early 2016 be considering options for procurement of both Radiology and Cardiology PACS function with an expectation that the PACS and EPR vendor will work together to provide an integrated view to our clinicians. 4.2.5 Genomics GOSH is a lead member of the North Thames Genomic Medicine Centre as part of the 100,000 Genomes Project1, as a result we have a rich database of genomic information that is held in a system that is a bespoke development for GOSH by Congenica Ltd This database should be connected to the Clinical / Business Intelligence and Research Platform. 1 http://www.genomicsengland.co.uk/the-100000-genomes-project/taking-part/genomic-medicine-centres/ Commercially Confidential Version 1.1 Page 14 of 72 Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation 5. EPR REQUIREMENTS 5.1 REQUIREMENTS OVERVIEW The Trust is currently developing an OBS (Output Based Specification) that describes what the Trust will expect to implement as part of the proven core EPR functionality it requires to be available from the Potential Provider (and any appropriate sub-contractors) on go-live. Updated versions of the requirements information presented below will be provided as part of the PQQ documentation if appropriate. The OBS itself will contain the detailed requirements of the Trust and will be issued to shortlised Potential Providers within the Invitation to Submit Initial Tenders (ISIT) documentation. This section now provides: 5.2 A summary of key scope and architectural issues A high level description of the Trust’s required functionality and the additional functionality the Trust is likely to want to deploy during the lifetime of the contract(s) A summary of Functional Requirements including examples of what is likely to be excluded from the contract(s) A summary of Non-Functional Requirements and Services The Trust’s indicative implementation timescales SUMMARY OF KEY SCOPE AND ARCHITECTURE ISSUES The selection of the right EPR for GOSH is not architecture-led but is solution-led in terms of functionality to support the clinicians’ work and the research ambitions of the Trust. The scope of the procurement is to provide the Trust with a highly integrated, but not necessarily a single database, EPR solution. For example, the Trust’s intention in this respect is to emphasise that, provided that all of the other Trust procurement criteria were to be satisfied to a very high quality, some or all of the following could feature as part of a successful procurement outcome for the Trust: Seamless integration of PAS functionality with wider clinical functionality Seamless integration of a limited number of Departmental and other systems with the Potential Provider’s core and highly integrated EPR solution Seamless clinical and business intelligence on the data in the EPR solution as a whole. The solution should be capable of expansion over time to replace the majority of the functionality currently provided by specialist clinical systems. The intention from day one is to deploy the core EPR system and incorporate the functionality of current specialist departmental systems, where possible, rather than replace them directly with new standalone departmental systems. For the avoidance of doubt, this procurement does not include the replacement of smaller specialist departmental systems except where specified in section 5.4.2. Commercially Confidential Version 1.1 Page 15 of 72 Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation The Trust requires the ability to discuss with Potential Providers how best to incorporate future optional requirements and replace legacy systems over time. Therefore, the Trust has selected the OJEU competitive dialogue procurement approach as it provides the widest possible market choice and the best opportunity to develop and refine solutions in dialogue with shortlisted Potential Providers. 5.3 HIGH LEVEL FUNCTIONALITY DESCRIPTION Leading on from the above, this section provides a high level description of the Trust’s required EPR functionality and the additional functionality the Trust is likely to want to deploy during the lifetime of the contract(s). The high level requirements for a solution are: The system will be selected and designed to support world-class paediatric care Intuitive and sophisticated user presentation of the entire patient record that can be used by the typical clinician with a reasonable level of training A resilient, highly available solution An advanced comprehensive EPR to contain administrative and clinical details of the entire patient pathway across all specialties and disciplines and to replace most of the disparate department and specialty systems currently in use Highly functional PAS suitable for use in the UK and compatible with all statutory and regulatory requirements with a commitment to remain compatible over the duration of the contract Electronic workflows and reports will support improved administrative and clinical processes and pathways Tailored specifically to the Trust’s paediatric requirements Comprehensive referral, waiting list, outpatient, inpatient, day case, outreach, and follow up management Protocols, best practice guidance and alerts embedded in the system will reflect latest world-wide research understanding of the most effective paediatric care Existing and demonstrable advanced evidence-based clinical decision support capability suitable for paediatric care and capable of full localisation and bespoke configuration Integrated electronic prescribing and medicines administration to increase the convenience and efficiency of the prescription-writing process Integrated comprehensive departmental systems for pathology, radiology, pharmacy and other departments providing diagnostic and treatment services Flexible configurability of product to allow design of the system by clinicians, patients and families, capable of capture of structured clinical and administrative datasets and annotation of diagrams A child friendly, age-appropriate Patient and Family Portal for use before, during and after interactions with the hospital Advanced self-service reporting tools will allow analysis of the structured data that will be in the record for operational management, clinical audit and management reporting Commercially Confidential Version 1.1 Page 16 of 72 Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation 5.4 Ability and support to integrate with legacy applications which are not replaced as required Wide compatibility with technology to improve efficiency and access to clinical systems everywhere, for example, workstations-on-wheels, mobile devices, digital pens, voice recognition and dictation systems and remote working, for example in outreach clinics High degree of integration with medical devices and diagnostic equipment The ability to share information electronically with care providers and stakeholders outside the Trust Conversion of data from existing systems into the EPR where necessary or into a linked, accessible historical data repository in the Vendor Neutral Archive The ability to manage, make available and use data from non-EPR systems inside and outside the Trust in varied standard formats (for example referral letters, outputs from specialist clinical systems like Audiology, Ophthalmology, Genetics) FUNCTIONAL REQUIREMENTS Our requirements are being developed by a set of Functional Groups made up of multidisciplinary representatives from the organisation. The requirements from all groups are a combination of requirements to replace existing solutions and requirements for improved, innovative solutions. 5.4.1 Core Requirements Please note that the numbers of requirements given in this table are to indicate the complexity and scope of the functional area and will not exactly match the final specification requirement numbers. Requirements in italics in this table are likely to be optional in the main EPR procurement to give GOSH the option of procuring them from specialist suppliers and interfacing these to the EPR. The Trust seeks to explore with suppliers the list of optional and core components in the Pre-Procurement Market Engagement exercise. Functional Area 1 People and Teams Commercially Confidential Version 1.1 Scope includes Patients Family members and relationships Staff Other professionals Roles Teams Page 17 of 72 Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation Functional Area Scope includes 2 Care Pathways and Decision Support The pervasive scope of pathways Evidence-based pathway modelling Participants in the pathway Content-driven pathways Shared pathways Early-warning and alerts 3 Patient Management Referral Equity of access Standardised referral channels RTT and other targets Internal referrals 4 Patient Management – Out Patients Clinic templates and set-up Patient booking and communications Outpatient diagnostics and therapy Clinic reorganisation Clinic outcomes 5 Patient Management – In Patients Waiting lists Bed management Workload management Admissions Discharge Planning Day-cases, ward attenders and out-patient therapy Critical Care Therapies 6 Theatres and Surgery Theatre scheduling Pre-Operative Assessment Surgical record-keeping Anaesthetics record-keeping Recovery Sterile services 7 Protecting the Child Confidentiality Access and Accountability Positive patient identification Consent Recording of concerns Child protection alerts Infection Control Allergies and other permanent alerts Collaboration with other agencies Commercially Confidential Version 1.1 Page 18 of 72 Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation Functional Area Scope includes 8 Record-keeping Human data entry Data from other information systems Medical device integration Charting Terminology Records management Legacy records 9 Information Presentation Ease and speed of access to information Context-driven views Navigation Aggregated and patient-specific Personalisation and configurability 10 Requesting and Fulfilment - General Order sets Requester workflow Fulfiller routing Requester-fulfiller dialogue Fulfiller workflow Outcomes of requests Requester follow-up 11 Requesting and Fulfilment - Drugs Prescribing Dispensing Administration Stock management Manufacturing 12 Requesting and Fulfilment - Pathology Ordering tests Specimen collection Pathology disciplines Specimen reception and tracking Laboratory workflow Genetic laboratories Results reporting Blood transfusion Infection control Commercially Confidential Version 1.1 Page 19 of 72 Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation Functional Area Scope includes 13 Requesting and Fulfilment – Imaging Requesting and order acceptance Appointment scheduling Radiology workflow Cardiac and other specialist modalities Modality integration Reporting Image archiving and VNA 14 Resource Management Single step scheduling People, Rooms and Places Tracking Demand and Capacity (inc. emergency capacity) Consumables 15 Leaving GOSH Long-term follow-up Transition to adult services Final discharge Long-term Outcomes 16 Specialty-specific requirements within the EPR Specialty functionality within core EPR Integration of specialty capabilities not available within the EPR Specialty development platform (in particular, for new services in the future) See Appendix 1. For a list of the Trust’s clinical divisions and departments. 17 Clinical and Business Intelligence Real-time business intelligence Data repositories and warehouses Data analysis and reporting tools Operational, financial and logistic information 18 Communications and Collaboration Production of clinical documents Multi-channel communications Electronic Document Interchange Portals Multi-Disciplinary Teams Shared care Cross-boundary pathways Transitional Care Clinical networks Commercially Confidential Version 1.1 Page 20 of 72 Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation Functional Area Scope includes 19 Master Data Management 20 Research and Trials See section 6.5 for the research-orientated capabilities that are likely to be provided by the core EPR system. 21 Platform People Terminologies Drug databases The mobile workforce Input technologies Identity and access management Security Interoperability Performance Availability Business Continuity Table 5-1 Core Functional Requirements 5.4.2 Excluded from EPR Procurement There are a number of systems that are considered to be outside the core and optional scope of this procurement (though some existing solutions will be interfaced or needed to support EPR). Suppliers should not respond with solutions in these areas: ICT Infrastructure services and technology, networking, endpoint devices, mobile solutions, etc. PACS Electronic Document and Records Management solution with scanning and records archiving except as integral to the VNA E-rostering Electronic staff records Supplies and stores management Business systems such as HR, finance, procurement Also, suppliers of single-specialty systems should not respond Commercially Confidential Version 1.1 Page 21 of 72 Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation 5.5 NON-FUNCTIONAL REQUIREMENTS AND SERVICES The Trust’s Non-Functional Requirements are summarised in the table below. Service Name Description Technical The technical platform which will underpin the Trust’s output-based requirements. Service Levels The services that are required to support and maintain the EPR solution. Implementation and Delivery The services that are required to support implementation of the EPR solution. Benefits Realisation The services that will be required to support realisation of the Trust’s target benefits. To complement the functional and non-functional scope set out above, the Trust will also be procuring a number of implementation-related and operational services. The Trust anticipates that there will be a number of ‘core’ services that will form part of the core scope of Potential Provider responses and envisages that this will include the following: 5.6 Project management of the implementation project Subject Matter Experts (SMEs)/product specialists Clinical and departmental workflow specialists Technical platform specialists Capability training for the GOSH EPR programme team Training, Train the Trainer and go live support Data conversion and archiving Interfacing and integration Second line service support provision Service Transformation, Cultural Change and Benefits Realisation INDICATIVE IMPLEMENTATION TIMESCALES In working with shortlisted Potential Providers to develop an implementation plan, the Trust is seeking to achieve a challenging, but realistic plan that considers, and balances, the following sometimes conflicting factors: The desire to realise benefits as quickly as possible The amount of organisational change that the Trust can reasonably sustain during the period whilst continuing to maintain patient safety The assured delivery of a safe and robust EPR solution The level of Trust, and other third party, resources required Commercially Confidential Version 1.1 Page 22 of 72 Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation The Trust will require shortlisted Potential Providers, using their experience and best practice methodologies, to work with the Trust during the Dialogue Stage to develop an appropriate implementation plan that will form the basis of Final Tender responses. This should address questions of risk management with respect to big bang versus phased implementation. In particular, the Trust wishes to emphasise the importance of support departments, such as pathology and radiology, to maintenance of its normal business across all specialties; and the need to ensure minimal disruption. The Trust also recognises the risks involved in implementing digital systems in outpatient settings, complex specialties, rare disease management, and outreach clinics, and the plan should recognise these risks. The implementation plan should take account of mandatory NHS-wide changes to requirements for information provision, interoperability and connectivity. Potential Providers will wish to note the following Trust starting points in respect of implementation and timescale issues: Contracting complete by Q4 2016 Implementation start early Q1 2017 Go live, appropriately phased to manage risk, in Q2/ Q3 2018 Commercially Confidential Version 1.1 Page 23 of 72 Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation 6. CLINICAL / BUSINESS INTELLIGENCE AND RESEARCH PLATFORM REQUIREMENTS 6.1 INTRODUCTION This section summarises the requirements for the Clinical / Business Intelligence and Research Platform to be procured as part of the EPR programme. Suppliers should note that in this area the requirements are less clearly defined and are likely to develop throughout the programme and the EPR contract period. The research platform will provide the basic analytics and the Trust seeks a partnership in which additional storage and analytic capabilities can be called off and stood down flexibly with demand and recognising budget constraints. 6.2 BACKGROUND With its broad range of clinical specialties, GOSH is in a unique position to undertake research into the most complex and rare childhood diseases. At any one time, there are several hundred active research projects ranging from observational studies to clinical trials of medicinal products. In collaboration with our colleagues at the Institute of Child Health, the Trust is currently involved with over 60 individual research groups dedicated to rare diseases. GOSH and the UCL Institute of Child Health2 were first awarded NIHR (National Institute Health Research) BRC (Biomedical Research Centre) status in 2007. A successful application in 2011 resulted in a further 5 years funding from 2012 to 2017. Find out more about our Biomedical Research Centre3 here. Our long-term commitment to instigating, supporting and delivering cutting-edge research means that every year a wide variety of around two hundred research projects are started, while at any one time there are roughly five hundred research projects active at the Trust. Research taking place at GOSH is structured around our six clinical areas: Critical Care and Cardio-Respiratory Diagnostic and Therapeutic Services Infection, Cancer and Immunity Medicine Neurosciences Surgery This work is strengthened by collaborative research conducted with teams from the five academic programmes of the UCL Institute of Child Health (ICH): 2 3 http://www.ucl.ac.uk/ich http://www.gosh.nhs.uk/research-and-innovation/gosh-biomedical-research-centre Commercially Confidential Version 1.1 Page 24 of 72 Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation Developmental Biology and Cancer Developmental Neurosciences Genetics and Genomic Medicine Infection, Immunity, Inflammation and Physiological Medicine Population, Policy and Practice Academics at ICH and clinicians at GOSH work together to continue our integrated and multi-disciplinary approach to the understanding, diagnosis, treatment and prevention of childhood disease. Since many staff hold appointments at both institutions, GOSH and ICH researchers are able to translate research undertaken in laboratories into actual trials in the hospital. We can ensure research offers a real benefit to children at GOSH, elsewhere in the NHS and internationally. GOSH is the lead member of the North Thames Genomic Medicine Centre as part of the 100,000 Genomes Project4 GOSH was a founder member of UCL Partners5, an Academic Health Science Centre for the region whose vision is to create a world leading centre for research, healthcare and education to deliver solutions to address the most pressing healthcare challenges for the health of our population. At any one time GOSH patients are taking part in hundreds of research studies and clinical trials to support a variety of research themes. Examples include: Molecular basis of childhood diseases: identification of disease-causing genes Diagnostics and imaging in childhood diseases: Development of our ‘Biomarker discovery’ approach incorporating genomics, proteomics, metabolomics and expression profiling, to maximise translational NHS hospital capability for new diagnostic tests Gene, stem and cellular therapies: driving therapeutic innovation in rare disease Novel therapies for translation in childhood diseases: exploiting our cohorts of unique and deeply phenotyped patients and stored biomaterial to develop and deliver novel experimental therapeutic interventions to treat childhood diseases The types of investigation and research undertaken include: 4 Quality improvement Operational efficiency Observational Studies such as efficiency in Theatres http://www.genomicsengland.co.uk/the-100000-genomes-project/taking-part/genomic-medicine-centres/ 4 http://www.uclpartners.com/who-we-are Commercially Confidential Version 1.1 Page 25 of 72 Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation 6.3 Effectiveness and efficacy of diagnostic techniques and of treatments Clinical studies Population studies using data from GOSH and collaborators Genomics and proteomics and their contribution to the understanding and treatment of childhood conditions Phenotype research Peer-reviewed studies of outcomes of treatments and techniques to create evidence Outcome studies and benchmarking Pharmacovigilance Personalised medicine, biomarkers and patient level prediction Disease Interception CLINICAL / BUSINESS INTELLIGENCE AND RESEARCH PLATFORM SCOPE This section describes the initial scope of requirements for clinical and business intelligence and to support the research activities of GOSH and its academic and health service partners, together with a summary of requirements as currently understood by the EPR Programme. The Trust’s requirements in this area fall into five main categories: 6.4 Clinical and Business Intelligence tools (see 6.4) to support the day to day running of the hospital, in terms of both clinical and support services, and to support performance management and planning over the longer term, as well as to satisfy the reporting obligations of the trust to a variety of external bodiesClinical trials (see 6.5) where the clinical activity carried out includes elements whose purpose is to provide information relevant to the aims of the study, including consent, cohort identification and management, trial enrolment and clinical trial activity management and to extract data to support charging. These requirements are expected to be met by the EPR Lot or the Research lot and are included as optional requirements in both lots – but we prefer to procure this as part of the EPR lot than separately Scientific administrative tools (see 6.6) to support research activities such as sample tracking; image analysis; GCP work to produce novel personalised therapies Analytics Platform (see 6.7) to support the sophisticated analysis of large quantities of data, over an extended period of time, over a wide population and derived from a wide range of systems managed by many organisations Translational research (see 6.8) providing support for clinical modelling, pre-clinical trials and clinical trials of diagnostic assays and equipment, medicines, healthcare equipment, computer systems, methods of treatment or molecular science CLINICAL AND BUSINESS INTELLIGENCE Although they have many characteristics in common, and may in due course share many tools and technologies, Clinical and Business Intelligence is a set of capabilities that can be distinguished from the research arena in several respects. Most important is that it supports Commercially Confidential Version 1.1 Page 26 of 72 Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation the day to day running of the hospital, both in terms of the delivery of clinical services and the support services on which the former depend. Clinical intelligence may detect an event relating to individual patient (and interact with the care pathway management functionality contained with the core EPR) or it may cross patient populations – a good example being the early detection of a pattern of infection, before the outbreak becomes apparent to any one clinician. Business intelligence is also of huge benefit to managers and planners, where the need may be real time (for example, the up-to-the-minute bed state), day-to-day (for example, staffing levels and waiting list management) or capacity and performance management over a longer timeframe, with the aim of fine tuning processes in order to improve performance, productivity and efficiency, or to acquire the capacity to meet long term changes in the demand for the Trust’s services The core requirements for provision of Clinical and Business Intelligence are as follows: The ability to satisfy mandatory reporting requirements, including those imposed by NHS England and the commissioners of the Trust’s services (which in many cases is also NHS England). Also included in this category are returns to the various clinical registries (e.g. The UK Renal Registry) to which GOSH contributes, and which are very demanding in terms of their complexity The ability to support the management of the Trust’s operations, both front-line clinical and support functions, with information derived directly from core EPR data sources. These will typically be called from within the transactional systems themselves, and be available to clinical staff in the course of their everyday jobs and include the ability to generate reports on groups of patients, single patients, and the ability to drill down from individual data points into the underlying transactions Support services that need accurate and relevant information include the finance department (for both financial and management accounting), our procurement services (particularly in respect of consumables such as drugs and theatre stocks), Quality and Improvement, ICT and Redevelopment. HR functions such as Training and Education and Performance Management are major users of business information. The Trust’s International and Private Patient’s service will consume the information needed for the billing and costing of the Trust’s extensive non-NHS activity While the ability to extract of information from underlying data sources is vital, it is also important that the Trust has the tools at its disposal to deliver data in ways that make its meaning clear to information users. In this regard dashboards and graphical presentation tools come to the fore Underlying effective Clinical and Business Intelligence, and indeed research activity is ability the ability to apply standard terminologies to structured and unstructured transactional data. Such terminologies include the mandated coding systems used by the NHS (ICD-10, IPCS, Reed etc., although the expectation is that the EPR project will expedite the move to SNOMED CT. The Trust is involved in a wide range of international research groups and disease registries, which often have their own terminology systems. Therefore there is a need to be able to code transactions under more than one such system and to map concepts between such systems. Commercially Confidential Version 1.1 Page 27 of 72 Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation While coding traditionally underpins much of NHS mandatory reporting, it is also important for data analysis where information objects data sources are loosely structured and not necessarily conformant with a consistent data model, or inherently unstructured but carrying metadata. Obviously, the ability to code transactions as an atomic part of their creation (rather than a retrospective exercise) will be critical to the success of a terminology-based approach to clinical recordkeeping At the further extreme of the continuum from highly structured to unstructured data lie radiological images, but even here clinical intelligence tools can be employed to detect patterns of interest within images. The benefits of employing such tools are threefold: efficiency – the sheer speed in which electronic systems can process data compared to highly expert humans (who in future will focus more upon exceptions than bulk data); accuracy – machines will be much more unlikely to miss something significant; the ability to detect correlations across wide ranges of data sources, for example the outputs of gene sequencing and proteomic analysis The means to extract precisely defined data sets for transformation and loading into intelligence-orientated data stores. The requirements in this regard are essentially the same as for the Analytics Platform described in section 6.7. Note that the question as to whether there should be separate stores for research and operational purposes is an architectural question for future analysis and decision Real-time intelligence, such that the impact of newly captured data will, within a very short time of its creation, be evaluated in combination with other information, for its clinical or operational significance. Note that this will work in combination with the general decision-support tools available within the EPR and may well make use of Complex Event Processing technology in order to achieve the highest speed of evaluation possible. A push mechanism will be the normal means of delivering such information to the appropriate user Although a differentiator between Clinical and Business Intelligence on the one hand, and Research platforms on the other is that the former will tend to be more specific to the Trust and hospital operations, it is also the case that under the former heading falls the need to contribute information to portals that are likely to be used to aggregate disparate data sources belonging to several organisations, and serving the needs of a wide range of users, with patients and their families being a particularly important group Alongside the ability to analyse historical data, and to detect and evaluate events as they happen, lies a third category, that of predictive analysis. The ability to combine historical trend data with forecasts of such things as population growth, microbial resistance to antibiotics, the weather and climate change (data that will typically be derived from external sources) will give the trust powerful tools for the planning of the capacity and configuration of services Finally, all the foregoing will take place against a backdrop of exponentially increasing scale: more types of data captured, more observations carried out and more data points per observation, more correlations to be sought, especially with Commercially Confidential Version 1.1 Page 28 of 72 Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation respect to genomic and proteomic data, and more interventions that require early detection of adverse trends 6.5 CLINICAL TRIALS FUNCTIONALITY FOR EPR USERS The capabilities required to support clinical trials will include: Recording the key research aims of each trial Recording the personnel involved in running the trial Recording whether the trial is being led by GOSH or another research institution Assistance in recruiting patients to trials by finding candidates within our existing (or past) patient population Alerting researchers when a patient meets the criteria for a trial but has not been asked for consent to take part Recording consent and managing it throughout its lifecycle, including applicability and currency of consent, limitations on consent, further consent needed Visibility throughout all clinical systems as to whether a patient is actively involved in a clinical trial The incorporation of research activities into pathway models, and the means to assign patients to research pathways rather than or as well as regular pathways Decision support tools that can take research activities into account when making recommendations about the direction of pathways The tagging of data (such as clinical notes and results) to indicate that it was obtained from a research pathway or from carrying out research-specific activity The ability to extract research-tagged data using simple queries or clinical and business intelligence tools The ability to download information on research activities for billing purposes It should be possible to identify commercially confidential information and protect it from viewing, especially when a trial is carried out in collaboration with a commercial organisation The production of case reports to record data on each trial subject during the course of the trial as defined by the protocol Researchers will also need to use EPR collaboration tools to facilitate communications with patients (and other involved parties) The detection of unexpected events (internal or external to the hospital) that may be relevant to the patient’s participation in the trial or may indicate a safety issue, for example an emergency admission or a referral to a new specialty (it must be possible to exclude some routing events from this alerting) The ability to identify and tag cohorts of control patients who meet the same criteria but are not enrolled in the trial Commercially Confidential Version 1.1 Page 29 of 72 Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation 6.6 SCIENTIFIC ADMINISTRATIVE TOOLS The main requirements for the provision of scientific administrative tools to support research activities are: 6.7 The ability to track samples taken for research purposes. For example, a blood sample might be taken of a patient with a particular condition (or one without that condition for control purposes). The sample could be aliquoted and stored for future purposes such as re-testing or testing of different techniques. A record of the samples in storage, their current status with regard to consent, which have been used and for what purpose, and which are still remaining for use The ability to tag images, samples, results, elements of notes, or other elements of the patient’s data as of research interest and for what reason. For example, it may be interesting to tag all the samples taken from patients with a rare disease or all of the images for those patients, regardless of whether the image or sample or image was diagnostic of the condition itself or taken as another part of the patient’s treatment. Then the samples and images and other EPR data fragments etc. could be used for research anonymously without recourse to the patient records The ability to store GCP preparation details of specially prepared drugs, autotransfusion material or other special preparations ready to be used for treatments. The eventual destination of a particular drug, material, or preparation should be stored The processing of extracts of data from the EPR to allow billing for activities that are purely for research purposes and are additional to clinical care such as additional tests, imaging studies, visits ANALYTICS PLATFORM The Analytics Platform is a collection of tools and technologies that will support the sophisticated analysis of very large and complex data sets, which over the next decade are expected to increase in scale by orders of magnitude. The diagram below is a conceptual architecture for the Analytics Platform, which will serve both the Clinical and Business Intelligence needs of the trust, and the requirements of it and its partners in the field of scientific and clinical research. Note that the diagram is intended to summarise capabilities rather than specific system components. In particular, the question of whether the demands of clinical and business intelligence on the one hand, and research on the other, are best satisfied by a common or different set of tools and data stores will be decided at a later stage of this procurement. Commercially Confidential Version 1.1 Page 30 of 72 Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation Figure 6-1 Possible Architecture of the Analytics Platform The main requirements in this area are as follows: The ability to extract rich transaction-level data sets from the GOSH EPR, as soon as possible after the data is created or captured. This stream of data may in principle be supplemented with data from other systems both within GOSH and external to the Trust The ability to stage, transform and load this data into a variety of data stores, as described below. This will include the means to link data from disparate sources over common identifiers (e.g. the NHS Number) A clinical data repository (CDR) containing copies of transactional data originating in the GOSH EPR but supplemented by trusted data from other sources. This data will underpin research activities but may also be consumed by decision support tools within the EPR itself It will be possible to protect the identity of patients (and other persons) without undermining the richness, quality and usefulness of data. This is likely to be provided by pseudonymisation tools and may point to the establishment of separate repositories for research and operational purposes. However, no decision on that question has been made at present and the trust is open to innovative suggestions on how these objectives can be achieved and reconciled There will be data warehouse capability whereby aggregated datasets will be available for reporting across the trust and, in principle, the wider child health economy. There will also be the capability to build and make available data marts for localised and departmental purposes. Note that he distinction made between the Commercially Confidential Version 1.1 Page 31 of 72 Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation CDR and data warehouse is intended to be conceptual and not to imply that the capabilities must be delivered by one, two or even more physical components As well as bringing data into the Analytical Platform environment, as described above, there will be the means to consume data in other external repositories. These are likely to be national (or international resources), where the scale and governance constraints make permanent import impractical, but the where the ability to use such data “on the fly” will be invaluable. Such resources could include the NHS PDS, HIEs, GP data repositories and XDS-based image and document networks The Analytics Platform will include terminology services such that all coding schemas and ontologies and in common use will be understood and cross-referenced. As well as the major nationally-endorsed systems (SNOMED CT and legacy coding systems) there will be the means to incorporate specialist ontologies that are typically much narrower in scope, but address much greater taxonomic depths and fineness of distinctions The data stores, and the tools whereby they are populated and queried must be capable of handling huge data volumes, and scalable to cope with the expected massive growth in data sets (e.g. genome and proteome data), and of transactions originating from an expanding range of medical devices and home monitoring equipment The means to use logic to connect disparate sources of data, for example the use of patient demographics to identify identity matches in external data The sources of data are highly unlikely to conform to a consistent data model so tools are required that can use a variety of sources in combination The use of fuzzy logic to connect disparate sources of data, for example patients with similar demographics but different spellings The means to search disparate data sources for research-relevant information including the ability to search for, display, and extract data using a range of filters, searches, sorting capability, etc. A variety of searching tools are required, such as database queries, text searches, joining data, filtering, etc. It must be possible for search for data using conditions on one or many structured data fields It must be possible to search for text, numerical values and other values in unstructured data fields It must be possible to search unstructured data for instances of textual and numerical data, using wild cards, regular expressions and Boolean operators. For example one might search for “cystic fibrosis” or “x result: n” where n is a numerical value and x is result identifier (and more complex such searches) It should also be possible to search unstructured data taking into account semantic concepts such as negation and the equivalence (or partial equivalence) of clinical terms Commercially Confidential Version 1.1 Page 32 of 72 Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation 6 It must be possible to extract data in industry-standard formats for further analysis (for example, CSV and the native formats of systems such as Excel, statistical analysis tools and research-specific solutions like RedCap6) It must be possible to apply data mining tools to our data stores in order to detect patterns that could lead to the identification of potentially fruitful research topics (as well as topics where research is already in progress) and also to improvements within the operational and logistic fields Population studies need not be concerned only with historic data sources – they may also consume newly occurring events It must be possible impose order on the information in order to make the data sets easier to handle. This may take place through several stages of aggregation and transformation It should be possible to save a set of data transformations, aggregations and ordering so that the same processes can be run repeatedly It should be possible to save searches so that they can be run with different selection criteria without having to rebuild the whole search There must be complete control over the access to data so that users are restricted to that data to which they have specifically been given rights to use and search It must be possible to restrict downloading of data to people with the rights to do so. The use of data for research purposes needs to remain in compliance with the current consent terms. This will be a combination of the original consent plus any changes that have been made. Compliance covers the type of data, the use of data and the timescale over which consent has been given. In particular, there should be a segmented data structure consent model such that individual researchers and groups of researchers can be given different access rights on different datasets, e.g. read, add, delete, transform, etc. For example the primary researchers for a dataset may be able to add, transform and delete their own data but only use the data extracted from EPR Similarly, the use and disclosure of data needs to remain compliant with any IPR constraints, for example, in the case of commercially sensitive data The Data Warehouse, Clinical Data Repository and any other data store must be technically independent of the EPR and its supplier so that the value of the repository can outlast the EPR contract Activities carried out to build, feed and analyse data in the data warehouse should have no effect on the performance of the EPR for clinical purposes. The segmented data structure and consent model should allow the joining of datasets using demographic fields but prevent researchers from seeing demographic and other patient identifiable data. This model should follow best practice guidance from the information commissioner’s office and should be compliant with the Data Protection legislation, guidance and regulations https://catalyst.harvard.edu/services/redcap/ Commercially Confidential Version 1.1 Page 33 of 72 Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation 6.8 TRANSLATIONAL RESEARCH Providing support for clinical modelling, pre-clinical trials and clinical trials of diagnostic assays and equipment, medicines, healthcare equipment, computer systems, methods of treatment or molecular science. GOSH supports pharmaceutical companies and medical device and equipment manufacturers by taking part in research that helps to define, develop and test novel therapies, diagnostics and medical devices. We also produce research that can be translated into ideas for novel therapies, diagnostics and medical devices. The requirements for the Clinical / Business Intelligence and Research Platform in this area are to support: Identification of areas where existing therapies are not producing the ideal outcomes Identification of potential populations for novel technologies Identification of rare diseases through molecular science Research into the use of the novel technologies in clinical practice Support for all phases of clinical trials of novel technologies Commercially Confidential Version 1.1 Page 34 of 72 Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation 7. IMPLICATIONS OF A LOT BASED PROCUREMENT 7.1 INTRODUCTION As part of this pre-procurement market engagement process, the Trust wishes to explore: The extent to which it is likely that Potential Providers will be in a position to deliver both a high quality EPR solution and a high quality Clinical / Business Intelligence and Research Platform; or Whether separate contracts with an EPR Potential Provider and with a Clinical / Business Intelligence and Research Platform Potential Provider are the more likely procurement outcome The extent to which some of the Trust’s requirements could potentially be delivered via Lot 1 and, also, via Lot 2 and, if so, how such a scenario would impact on the procurement For the avoidance of doubt: The Trust recognises that some of its Clinical and Business Intelligence needs (per 6.4) may be met by the EPR platform directly. Similarly the Trust recognises that some of the requirements summarised in section 6.5 (Clinical trials functionality for EPR users) are likely to be met by the EPR platform (for example extending care pathways for research orientated tasks and observations), whereas other (for example, the overall case management of a trial) may not The Trust does not wish to enter into a sub-contracting arrangement for the delivery of the other elements of the Clinical / Business Intelligence and Research Platform Rather, the Trust is clear that it wishes to contract directly and flexibly with a Potential Provider or Potential Providers to deliver the Clinical / Business Intelligence and Research Platform The primary reason for doing so is that the Trust wishes to have flexibility as to how much business intelligence and research capability it makes acquires, and at what time, and to be able to do that affordably and flexibly, possibly on a different timescale to the EPR implementation Secondly the Trust wishes to enter directly into a partnership based relationship with a Clinical / Business Intelligence and Research Platform Potential Provider as opposed to being required to manage that relationship on an arms-length basis via another party Thirdly the Trust’s instinct is that different contract forms would be required for EPR, as opposed to the Clinical / Business Intelligence and Research Platform but recognises that contractual agreements such as collaboration agreements may be needed Fourthly the Trust recognises that different elements of the Clinical / Business Intelligence and Research Platform could come from more than one supplier including the EPR supplier and wishes to retain the flexibility to contract with more Commercially Confidential Version 1.1 Page 35 of 72 Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation than one supplier for the Clinical and Business Intelligence and Research Platform lots It follows that the EPR and the Clinical / Business Intelligence and Research Platform contracts would only be awarded to the same Potential Provider were that Potential Provider successful in bidding for each lot in its own right. 7.2 IMPACT ON THE PROCUREMENT PROCESS The Trust considers that its EPR and Clinical / Business Intelligence and Research Platform requirements are very much intertwined and that, were two contracts with separate Potential Providers to result, those contracts would need to reflect a series of appropriate interdependencies and ‘hand-offs’ in order to underpin the effective Trust management of the inevitable contract interfaces. It follows that the Trust is prepared to, in effect, run two parallel EPR and Clinical / Business Intelligence and Research Platform procurements, to very similar timescales, in order to ensure that the: Dialogue Sessions to be held with the Potential Providers participating in each Lot address any issues that impact on the other Respective contracts are completed at the same time That said, the Trust recognises that aspects of the EPR and Clinical / Business Intelligence and Research Platform procurements are likely to be different. For example: The Trust’s EPR requirements, as will be expressed in the OBS, are relatively firm Some parts of the Business Intelligence solution are likewise relatively firm However, much of the full specification for the Clinical / Business Intelligence and Research Platform procurement is likely to be largely developed in the course of the Dialogue process The EPR procurement is, therefore, likely to require different Dialogue Sessions to those for the Clinical / Business Intelligence and Research Platform procurement Commercially Confidential Version 1.1 Page 36 of 72 Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation 8. BUDGET, CONTRACT AND POTENTIAL PROVIDER ISSUES 8.1 INTRODUCTION This section provides indicative information on the Trust budget, contract and Potential Provider issues. This indicative information is offered for Potential Provider consideration and discussion as part of pre-procurement market engagement. Where appropriate, further and updated Trust thinking will be reflected in the version of this paper to be issued with the PQQ documentation. 8.2 TRUST BUDGET The Trust has agreed an Outline Business Case budget for supplier costs of approximately £17m. It should be recognised that this budget was based on early indicative costings and that, though affordability of the solution is extremely important, this does not represent a cap for the procurement. Rather, the Trust intends to assess Total Costs of Ownership, together with: the quality of solutions the confidence of delivery of the solution and associated benefits the innovative nature of the offering, and any additional benefits that the Trust had not foreseen but may accrue the risks to the Trust In the light of Potential Provider responses to the Trust’s request for indicative pricing set out at Appendix 1, the Trust will revisit its Outline Business Case cost assumptions and may revise the scope of the Clinical Research and Business Intelligence or the EPR accordingly. The Trust is unlikely to set a firm budget for the procurement before the Dialogue stage of the procurement. 8.3 TCO APPROACH In assessing Potential Provider TCO offers, the Trust will take considerable care at the Final Tender stages of the procurement to ensure that it is evaluating Potential Providers on a like for like basis over the entire expected period of the contract. For example, Initial Trust thinking is that this might reflect some or each of the following: Potential Provider costs associated with the solution to be implemented at go live Potential Provider Road Map costs and/or any costs associated with subsequent enhancements/releases Potential Provider costs associated with specific Trust paediatric developments Potential Provider costs that may be additional to the core costs of the solution, for example for additional services or licences The sensitivity of the costs to information provided by the Trust or the Potential Providers and our assessment of the reliability of that information Commercially Confidential Version 1.1 Page 37 of 72 Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation Trust costs including personnel, opportunity costs, procurement costs for parts of the solution not provided by the main EPR supplier Third party supplier contract costs Potential Providers will wish to note that, if necessary, the Trust will require them to include a paediatric development budget within their Initial and Final Tenders. The purpose of such a budget would be to cover any Potential Provider costs associated with developing Potential Provider solutions in order to satisfy the Trust’s core paediatric requirements. 8.3.1 Innovative Tenders The Trust wishes to emphasise that it would very much welcome innovative Potential Provider Initial and Final Tenders. Please note that Initial and Final Tenders will receive credit for any optional or other functionality or services of value that: 8.4 Are provided at no additional acquisition cost to the Trust alongside, or as an integral part of, other functionality Are provided on the basis that the value of the benefits to be derived from its implementation would demonstrably and significantly exceed the associated acquisition and implementation costs HOSTING The Trust is clear that it wishes to have a direct relationship with its hosting provider. Accordingly, the Trust’s current thinking is that it is likely to run a framework based procurement for hosting services based on initial tender information on the Warranted Services Environment. 8.5 PAYMENT PROFILE As yet, the Trust does not have a firm view surrounding the payment profile approach that it wishes to adopt. Rather, the Trust is likely to want to discuss the following options with shortlisted Potential Providers at the Dialogue stage: 1) A ‘traditional’ capital/revenue split that balances Potential Provider cash flow with the need for a Trust/s to stage payments in line with key implementation plan deliverables 2) A flat ‘managed service’ type profile apportioned over a 10 year period 3) A benefits realisation related profile that reflects Trust cash flow 4) A hybrid approach involving initial Trust payments linked to key deliverables and, thereafter, further payments linked to benefits realisation 8.6 THE ROLE OF BENEFITS IN THE PROCUREMENT The Trust’s benefits approach reflects: The Transformational nature of this procurement Commercially Confidential Version 1.1 Page 38 of 72 Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation The fact that the Trust is seeking a partner to work with in transforming and improving the way it delivers services to patients and carers The requirement for the successful Potential Provider’s benefits proposals to contribute to the Trust’s affordability assessment to be made in the Full Business Case In recognition all of the above, the Trust will be seeking more detailed Initial and Final Tender benefits and benefits realisation proposals compared to those that they understand are typical for EPR procurements. As part of doing so, the Trust will be seeking a combination of cash releasing savings benefits and more qualitative, non-cash-releasing, benefits. Given the Trust’s benefits focus, the conduct of procurement will address the extent to which Potential Provider solutions, as evidenced by OBS responses, Solution Demonstrations/Verification and Reference Sites, will support the proposed benefits case studies to be presented in Initial and Final Tenders. The Trust intends to use Reference Site visits and teleconference calls to explore the actual benefits obtained compared to the reference sites’ expectations. The Trust’s thinking surrounding the process re the above is set out in Sections 9.6.3 and 9.8.4. This content is offered for discussion with shortlisted Potential Providers at the very outset of the Initial Tender stage of the procurement. 8.7 POTENTIAL PROVIDER PROFILES The Trust wishes to contract with an EPR Potential Provider meeting the profile set out in the following table: Commercially Confidential Version 1.1 Page 39 of 72 Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation Qualifier ‘Ideal’ Trust Potential Provider Profile Reference sites Multiple reference sites for similar core ‘enterprise-wide’ clinical EPR requirements, other core requirements and optional requirements in healthcare organisations of a similar size and nature, ideally of a specialist paediatric nature Highly effective use of a tried and tested PAS that ideally fully satisfies the requirements of the Trust’s funding bodies (Please note that the Trust is not prepared to work with a Potential Provider to ‘Anglicise’ a PAS that is substantially or partially unproven in respect of the requirements of the Trust’s funding bodies) Full or near-to-full coverage of core requirements Significant coverage of optional requirements The Trust does not wish to adopt a ‘best of breed approach’ The Trust is, however, content to receive proposals involving limited, near to seamless, integration between, for example, the proposed PAS and the wider ‘enterprise-wide’ EPR solution, or, between the EPR and a relatively limited number of ‘departmental’ solutions Functionality High compliance with functional requirements Prime Contractor The prime contractor for the EPR would be the main application provider for PAS and core clinical functionality The prime contractor for the EPR would ideally be the prime contractor for core modules of the solution such as Pathology and Radiology. The Trust would consider separate procurement of a limited set of such departmental systems but would factor in the costs of doing so to the TCO calculation Confidence in provider’s ability to deliver on time, budget, and to the required standard Ability to accurately specify required Trust resources Confidence in the providers’ ability to increase and maintain Trust capabilities Benefits/Transformation Able to reference strong benefits realisation from existing customers Able to reference strong benefits return on investment (RoI) and cash releasing savings from existing customers Support for Trust benefits realisation and transformation Solution Implementation Commercially Confidential Version 1.1 Page 40 of 72 Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation Qualifier ‘Ideal’ Trust Potential Provider Profile agenda Technical Tried, tested, proven and referencable technical solution(s) A clear methodology for advising the Trust on the technical platform requirements Use of mobile devices for onsite and remote working, ideally including disconnected working Flexible and future-proofed The Trust wishes to contract with a Clinical / Business Intelligence and Research Platform Potential Provider meeting the profile set out in the following table: Qualifier ‘Ideal’ Trust Potential Provider Profile Reference sites Ability to demonstrate the use of its products in healthcare Ideally, the ability to reference sites similar in nature to the Trust (for example, with a strong genomics element to its business) Innovative, flexible solution Ability to increase and decrease additional (and especially research) solution functionality as required Ability to increase and decrease data volumes and types flexibly as required Innovative solutions offering exciting potential for world class clinical and business intelligence and research Clinical data repository containing all the EPR data Proven solution for clinical intelligence to support the clinical management of a specialist hospital Proven solution for business intelligence to support the operational management of a specialist hospital Data warehouse for Trust and external data Innovative analytics tools and solutions Innovative functionality to support scientific research One or several suppliers may be chosen to work in partnership to satisfy the requirements of this lot The EPR lot supplier may be one of these suppliers and EPR suppliers are welcome to bid for all or part of this lot A prime contract arrangement with subcontracting for Solution Functionality Prime Contractor Commercially Confidential Version 1.1 Page 41 of 72 Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation Qualifier ‘Ideal’ Trust Potential Provider Profile specialist functionality or services is a possibility for this lot (e.g. a prime supplier with solutions working with a subcontractor with specialist knowledge of implementing those solutions in healthcare, or a prime contractor with part of the solution working in partnership with a subcontractor with other parts of the solution) Ideally, reference-able experience of implementation of the solution in healthcare but this is not essential for truly innovative solutions Risk-sharing to protect the Trust where innovative solutions, novel in healthcare, are proposed Benefits/Transformation Proven ability to enhance the clinical management of the Trust to deliver quality benefits Proven ability to enhance the operational management of the Trust to deliver productivity, efficiency and financial benefits Proven ability to enhance research operations (measured by peer-reviewed, cited publications) Open platform that is vendor neutral Open platform to allow the use of third party analytical tools A solution which will allow the Trust to make use of its data independently of the chosen EPR and beyond the life of the contract for the EPR Implementation Technical 8.8 CONTRACT DURATION AND FORM The Trust intends to award a ‘base’ ten year contract(s); the contract(s) will incorporate appropriate break clauses. Contract(s) extension provisions are anticipated to apply for a minimum of a further five years, and potentially for significantly longer. Decisions surrounding potential contract(s) extensions would be informed by appropriate price and service benchmarking. A tailored version of HM Treasury’s Model Services Contract (MSC) will be used as the basis for the procurement. The MSC is a technology contract form specifically designed for the complexity and risk associated with contracts in excess of £10m. Because the MSC is a generic technology contract, our procurement advisers (High Resolution Consulting) worked with Mills & Reeve LLP, who developed the NHS Standard Terms & Conditions on behalf of the Department of Health, to develop a customised version of the MSC to reflect the contract terms required of an NHS EPR contract. Potential Provider acceptance of this overall contract(s) approach will be a pass/fail PQQ condition. Commercially Confidential Version 1.1 Page 42 of 72 Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation 8.9 CONTRACT SCOPE AND POTENTIAL CONTRACT ADDITIONS In addition to the requirements set out above, the Trust will reserve the right to add other, as yet unspecified, EPR, Clinical / Business Intelligence and Research Platform and IM&T related requirements to the contract(s) over time should they wish to do so. This would only occur where the prime contractor demonstrates the necessary capacity and capability to deliver value for money solutions and services to required timescales. The Trust has, therefore, defined a relatively broad contract scope in the Contract Notice and in all other procurement documentation so as to legitimately allow for any such additions. Equally, the Trust reserves the right to source, for whatever reason, elements of the additional optional requirements from alternative Potential Providers. This may occur should the successful prime contractor fail to provide: A competitive offer for any or all of these optional requirements via the procurement, or via contract change control; or Effective functionality, integration or value for money in delivering core requirements For the avoidance of doubt, it follows that the Trust will not accept any contractual obligation to use the selected prime contractor for any process or system additions via the optional requirements detailed above, nor for any other requirements falling elsewhere with the broad contract scope to be drawn. Nor will the Trust accept any contractual penalties should it opt to source any of the Requirements from elsewhere. The version of this paper to be issued with the PQQ documentation will provide further detail on the Methodology for Potential Contract Scope Additions. 8.10 KEY COMMERCIAL PRINCIPLES A set of standard commercial principles traditionally used to underpin a procurement of this nature are intended to be presented in the next version of this paper. However, in the light of the key aim to establish a long-standing and genuine partnership via the procurement, the Trust does not consider a ‘standard’ EPR procurement approach to commercial principles to be entirely appropriate in these circumstances. For example, this paper has already noted in Section 3 the Trust’s willingness to consider entering into a shared, collaborative, partnership based contract. The Trust is also interested in other innovative approaches that the supplier can offer in order to offset risks and improve the short-term and long-terms success of the programme. The precise structure of any such contract that reflects the Trust’s Key Commercial Principles will be the subject of Dialogue with the shortlisted Potential Providers to receive the ITPD. Shortlisted Potential Providers will, therefore, be invited to discuss with the Trust what they consider to be an appropriate approach to commercial principles for this opportunity. In Commercially Confidential Version 1.1 Page 43 of 72 Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation advance of that, Potential Providers will be invited to mark-up the Trust’s principles at the Initial Tender stage and to reflect their commercial principles negotiating position in their ISIT responses. Detailed clarifications and negotiations will then take place with shortlisted Potential Providers during the Dialogue stage. For its part, the Trust will be seeking to agree a commercial principles approach that balances the partnership nature of the relationship but which also provides the Trust with an appropriate level of recourse to penalties in the event of persistent poor performance. Commercially Confidential Version 1.1 Page 44 of 72 Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation 9. CONDUCT OF THE PROCUREMENT 9.1 INTRODUCTION This section provides indicative information on the detailed conduct of the Procurement. This indicative information is offered for Potential Provider consideration and discussion as part of pre-procurement market engagement. Where appropriate, further and updated Trust thinking will be reflected in the version of this paper to be issued with the PQQ documentation. 9.2 INDICATIVE PROCUREMENT TIMETABLE Set out below are the key dates in the proposed procurement timetable. Please note that this timetable is intended as a guide and that the Trust reserves the right to revise it at any time. In particular, the Trust reserves the right to vary this proposed timetable in response to: Potential Provider feedback at, or arising from, the Potential Provider Briefing Event The need to balance the proposed procurement timescales with the availability of the Trust’s resources whilst ensuring the continued delivery of the Trust’s day to day clinical and operational priorities Potential Provider feedback and progress made in the conduct of the procurement, in particular during Dialogue sessions The need to balance the proposed duration of benefits realisation activity and of the Dialogue stage of the procurement with the need to allow Potential Providers and the Trust’s sufficient time to develop and discuss benefits realisation proposals For the avoidance of doubt, the Trust reserves the right to: Extend the Dialogue stage of the procurement to allow all shortlisted Potential Providers reasonable time to develop their Final Tender proposals in conjunction with the Trust If necessary, allow one or more Potential Providers to spend longer than others to complete their proposals in conjunction with the Trust (on the basis that some Potential Providers might require more time than others to fully develop their proposals and that it would be in the clear interest of the Trust to receive fully developed proposals from all that maximise the procurement outcomes for the Trust) It follows that the latter stages of the indicative procurement timetable presented below are less firm compared to the earlier stages. Commercially Confidential Version 1.1 Page 45 of 72 Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation MILESTONE TARGET DATE Stage 1 – Pre-Qualification Place Contract Notice & make PQQ Documentation Available 29/01/2016 PQQ Response Deadline 29/02/2016 PQQ Short listing (target no more than 5) 18/03/2016 Stage 2 – Initial Tender Invitation to Submit Initial Tenders Issued 18/03/2016 Submit ISIT Responses 13/05/2016 ISIT Short listing (target no more than 3) 24/06/2016 Stage 3 – Dialogue Invitation To Participate in Dialogue (ITPD) Issued 27/06/2016 Solution Verification/Benefits Sessions Over the period 11/07/2016 – 02/09/2016 Reference Site Visits and Conference Calls Over the period 11/07/2016 – 02/09/2016 Dialogue Ends 02/09/2016 Issue Invitation to Submit Final Tender (ISFT) 05/09/2016 ISFT Response Deadline 19/09/2016 Preferred Potential Provider Award 03/10/2016 10 Day Standstill Period Ends 13/10/2016 Stage 4 - Contract Award FBC Approval 30/11/2016 Contract Award 01/12/2016 Table 9-1 Indicative Procurement Timetable 9.3 PROCUREMENT STRATEGY REQUIREMENTS 9.3.1 Introduction The Trust’s procurement strategy is specifically designed to maximise the prospects of identifying the ‘right’ Potential Provider(s) with which to establish a genuine and longstanding partnership based relationship to deliver its requirements. The Trust understands that the majority of partnership related issues can only be effectively and substantively Commercially Confidential Version 1.1 Page 46 of 72 Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation addressed during the Dialogue stage of the procurement with shortlisted Potential Providers, as opposed to at the PQQ or Initial Tender stages. The Trust’s planned approach to Potential Provider ‘down-selection’ during the procurement process is set out later in this section of the paper. Please note, however, that the Trust reserves the right to de-select (at any time during the procurement) any Potential Provider failing to adhere to any aspect of the PQQ declaration, for example, by failing to exhibit the partnership based working principles referred to throughout this paper. 9.4 PROCUREMENT TIMETABLE – COMMENTARY A more detailed commentary designed to ‘bring to life’ the key actions and the key outcomes associated with each of the 4 Procurement Stages is provided below. Whilst not required for PQQ response purposes, this additional detail is provided in order to inform Potential Provider decision making about whether to bid for this exciting opportunity. This explains that a key feature of the planned conduct of the Initial Tender and Dialogue stages is to increasingly develop and agree populated contract schedules, to the point that near-tosignature schedules, acceptable to the Trust, are in place prior to the issue of the ISFT. 9.5 STAGE 1 - PRE-QUALIFICATION The Pre-Qualification stage commences with the simultaneous issue of: An OJEU Contract Notice A PQQ Requirements and Completion Instructions document A PQQ Response document PQQ Scoring Criteria Descriptions This Potential Provider Briefing Paper (updated as necessary in the light of preprocurement market engagement at, and around, the Potential Provider Briefing Event) It is important to note that much of the content of this background information paper and descriptive document is not intended to contribute to, or to inform, the PQQ process. Rather, the majority of the content of the paper comprises information to feature in the subsequent Invitation to Submit Initial Tender (ISIT), Invitation to Participate in Dialogue (ITPD) and Invitation to Submit Final Tenders (ISFT) documentation. The early provision of this content is designed to inform Potential Provider decision making about whether to submit a PQQ response. Please note that the Trust will issue a detailed Output-Based Specification to shortlisted Potential Providers with the Invitation to Submit Initial Tender (ISIT) documentation. Following receipt of PQQ responses, Stage 1 concludes PQQ response evaluation, Potential Provider shortlisting (ideally, no more than 5 for each lot), and issue of PQQ outcome letters. Please note that the Trust reserves the right to reconsider the design of the procurement, and the number of procurement stages to be followed, in the event that it Commercially Confidential Version 1.1 Page 47 of 72 Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation were to receive a relatively small number of convincing PQQ responses from Potential Providers with a close match to the Trust’s target Potential Provider profile. 9.6 STAGE 2 – INITIAL TENDER 9.6.1 Introduction The Trust will issue an Invitation to Submit Initial Tenders (ISIT) to Potential Providers shortlisted following PQQ evaluation. Following initial discussions with the Trust, shortlisted Potential Providers will be invited to commence work on their Initial Tenders. Initial Tenders are likely to comprise: An Overview Innovation Response to a range of Trust functional and non-functional requirements Benefits proposals Pricing and commercial proposals Please note that each aspect of these ISIT responses will be the form of contributions to draft contract schedules which will be supplemented throughout the Dialogue stage of the procurement in building towards to the ‘near to signature’ contracts that will form the majority of Potential Provider Final Tenders. 9.6.2 Functional and Non-Functional OBS responses Potential Providers will be asked to provide responses to the Trust’s OBS and ‘self-score’ their compliance. On receipt of Potential Provider responses, the Trust will undertake a desk-based exercise to re-score and raise clarification points on the responses with the three shortlisted Potential Providers during the Dialogue Stage. This Trust’s feedback will form the basis of the OBS Functional & Non-Functional clarification sessions. This clarification process will continue as required, on a basis to be agreed with each shortlisted Potential Provider, throughout the Dialogue Stage, in parallel to other activities. The clarification process will conclude prior to closure of the Dialogue phase and the issue of the Invitation to Submit Final Tenders (ISFT). 9.6.3 Scoping and Preparation of Initial Tender Benefits Responses The Trust’s current thinking surrounding the process re the above is set out below: Potential Providers will be required to provide 5 reasonably detailed cash-releasing and 5 non-cash-releasing benefits and benefits realisation case studies as part of Initial Tenders. Those Initial Tenders may comprise some of the potential benefits areas identified by the Trust and/or additional benefits areas identified by Potential Providers. The Trust will issue its benefits starting point to shortlisted Potential Providers with the Invitation to Submit Initial Tender (ISIT) documentation; Commercially Confidential Version 1.1 Page 48 of 72 Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation Each case study should detail the: o Readiness position that the Trust would ideally be in, or be working towards, in order to realise the specified benefits o Required Trust’s readiness action (where the Potential Provider knows, or might surmise, that the Trust still have some way to go in terms of preparation) o Specific actions (over and above readiness preparations) that would be required to be undertaken in order to realise the specified benefits. Those actions should be broken down into Potential Provider, Trust side and jointworking responsibilities The Trust will evaluate those Initial Tenders. In doing so, it will consider the implementation proposals made by Potential Providers and allocate an initial associated benefits realisation confidence ratings (scores) to those proposals. Case studies will be scored on a qualitative basis according to the relative ambition that they exhibit and the confidence associated with them. (The confidence ratings will be updated after Dialogue and Reference Site visits and teleconference calls). 9.6.4 ISIT Response Arrangements The ISIT is likely to have a 56 day response deadline. Following receipt of Initial Tenders, Stage 2 concludes with Proposal evaluation, Potential Provider short-listing (ideally, no more than 3 per lot), and the issue of ISIT outcome letters. 9.7 STAGE 3 - DIALOGUE 9.7.1 Introduction The Dialogue Stage commences with the issue of an Invitation to Participate in Dialogue (ITPD) to the shortlisted (ideally no more than 3) Potential Providers. The Trust’s starting point for the procurement is that it will be rooted in a core of relatively firm Functional Requirements, especially for the EPR lot. However, the Trust is conscious that aspects of its world-class paediatric and the Clinical/Business Intelligence and Research requirements are relatively niche in nature and that the options for satisfying these requirements in particular will be a matter for discussion during the Dialogue stage of the procurement. The Trust also assumes that all of the following will be open for negotiation as the Trust seeks to strike the right balance during Dialogue between quality, delivery standards, delivery assurance and price: Outcome delivery and assurance System ‘keep or replace’ decisions Implementation phasing and timescales Project and programme management Working in partnership Supporting transformation Commercially Confidential Version 1.1 Page 49 of 72 Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation Benefits proposals and realisation Risks to the Trust’s business and income Confidence in the TCO calculations Commercial issues Non-Functional Requirements The Trust is exploring how best to strike the correct Dialogue stage balance between the issues that are genuinely for discussion and negotiation as opposed to those issues that are primarily for clarification surrounding many of the Trust’s Requirements and Potential Providers’ responses to them. Each of the main elements of the Dialogue stage, and the main issues associated with them, is explored in further detail in the following sections. 9.7.2 Dialogue The primary focus of the Dialogue sessions is to ensure that both the Trust and Potential Providers have the opportunity to exchange thoughts and initial starting points surrounding requirements and proposals and to discuss those issues, in a constructive environment. The Trust will present their feedback on Potential Provider Initial Tenders. Potential Providers will present their further thinking surrounding how they propose, during the Dialogue stage, to refine their thinking surrounding the delivery and support of the Trust’s EPR-enabled transformation programme, deliver an impressive benefits package and respond to the key Commercial Principles. With the above in mind, the focus of dialogue sessions will be to allow for the necessary refinements, clarifications and discussions surrounding Proposals on the following key issues culminating in mature final draft Terms & Conditions and Contract Schedules: The approach to contracting for functionality that the prime EPR supplier does not provide and is proposing a prime contracting arrangement, or that the Trust retains existing modules, or that the Trust contracts for new specialist modules, and the systems integration relationships involved The best means for provision of optional functionality such as specialist departmental functionality and VNA The intersections between the functionality in the two main lots and the best means to achieve a highly functional but value-for-money set of EPR and research data warehouse and analytics tools Implementation & delivery, including phasing, testing and milestone acceptance, transition issues and respective responsibilities etc. Benefits and benefits realisation, including any innovative proposals Approach to technical platform solution including service levels, service risk management, collaboration with hosting suppliers, information governance, security issues, integration etc. Commercially Confidential Version 1.1 Page 50 of 72 Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation Service management and service levels, including help desk, incident and problem management, respective responsibilities and the trade-off between service levels and price etc. Financial offer, including charging and invoicing, milestone payments, capital/revenue split, the proportion of deployment payments to be paid after ‘golive’; the level of poor performance remedies etc. Commercial offer and proposed Agreement mark-up Given the potential for Potential Provider benefits responses to be very different, the Trust may need to spend different amounts of dialogue time on different aspects of dialogue with Potential Providers. It follows that the latter stages of the indicative procurement timetable presented above are less firm compared to the earlier stages, in part due to the fact that the Trust reserves the right to allow one or more Potential Providers to spend longer during the Dialogue stage than others to complete their Final Tender benefits proposals in conjunction with the Trust. 9.7.3 OBS Functional and Non-Functional Requirements The Trust’s Initial Tender feedback will form the basis of face to face OBS Functional and Non-Functional clarification sessions to be held with each with Potential Provider. This clarification process will continue as required, on a basis to be agreed with each Potential Provider, throughout the Dialogue Stage, in parallel to other activities, and will conclude prior to issue of the Invitation to Submit Final Tenders (ISFT). 9.7.4 Dialogue Stage Closure Before closing the Dialogue stage of the procurement, the Trust will need to be satisfied that the outcomes of the Dialogue Rounds will ensure that: Final Tenders will comprise a robust contractual basis Final Tenders will be capable of acceptance Final Tenders will reflect a genuine meeting of minds that we have together established during the Further Dialogue Rounds Only post Final Tender clarification, as opposed to negotiation, would be required Once the Trust is content that only post Final Tender clarification, as opposed to negotiation, would be required, Dialogue would close, followed by the issue of the ISFT. However, as already noted, the Trust reserves the right to extend the Dialogue Stage should it become clear that sufficient progress is not being made to achieve the required level of completeness of ISFT responses. Please note that: ISFT response must be final and not subject to change or renegotiation, although Potential Providers should note that clarification is likely to take place with all Potential Providers to ‘clarify, specify and optimise’ Tenders, as set out at 30(17) and 30(18) of the Public Contract Regulations 2015 Commercially Confidential Version 1.1 Page 51 of 72 Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation Potential Providers will be required to agree, as part of Final Tenders (and any variants therein), that any post-Final Tender clarifications, confirmations and/or supplements will not result in any price increases, service level degradation, risk transfer, transition timescale delays or any other similar or equivalent variations that might erode the agreed position of the Trust, or change the economic balance that we will have together established through the Dialogue stage It is a requirement for the ISFT response to be based on the solution(s) and responses that were presented in your PQQ and ISIT responses and during the Dialogue Stage 9.7.5 The Potential for Potential Provider ‘down-selection’ during the Dialogue stage The Trust does not plan to include a formal ‘down-selection’ process designed to reduce the number of Potential Providers participating in the procurement. Ideally, all three shortlisted Potential Providers will participate in a highly competitive procurement from the issue of the ITPD until Final Tender stage. That said, the Trust does consider that it would own a ‘duty of care’ to advise any Potential Provider were they to be falling significantly behind their competitors during the Dialogue stage. The main potential scenarios that the Trust can envisage might arise are briefly discussed in the following paragraphs. 9.7.5.1 Total Cost of Ownership (TCO) The Trust will not, as is the case for some procurements of this nature, be setting a price limit above which Initial Tenders or Final Tenders would be excluded from the procurement. The Trust plans to: Discuss the means for evaluating TCO with Potential Providers as part of preprocurement market engagement in the light of Potential Provider responses to the Trust’s request for indicative pricing for a world-class EPR (see Appendix 1) Set out its resulting thinking, including the indicative Potential Provider and TCO budgets, in the updated version of this paper to be issued as part of the PQQ documentation This said, Potential Providers should note that: Should it need to do so, the Trust would be prepared to revisit the appropriateness of its indicative Potential Provider and TCO budgets as the procurement progresses Ultimately, however, proposals reflecting an unaffordable price, or a price that does not provide value for money or a Final Tender that passes too much technical, commercial or other risk to the Trust, will not be successful The Trust also reserves the right not to award a contract to any Potential Provider 9.7.5.2 Functional Requirements The Trust’s core principle is to procure a highly functional solution to deliver stated requirements and this is likely to be reinforced via an approach along the following lines Commercially Confidential Version 1.1 Page 52 of 72 Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation that is likely to be applied to the Trust’s consideration of OBS responses on their initial receipt during the Initial Tender stage and, also, at the Final Tender evaluation stage. The Trust is likely to set an acceptability threshold score as a % of the total available compliance score. That threshold would reflect the Trust’s view regarding what constitutes an acceptable functional requirement score. This threshold would not represent an automatic pass/fail OBS evaluation condition, whereby any Potential Provider failing to reach that minimum functional requirement score would be excluded from the competition. However, the Trust would reserve the right to de-select any or all Potential Providers failing to meet this unacceptability threshold. The potential for this scenario to arise would depend on the quality of OBS, ISIT and Final Tender responses received. 9.7.5.3 Other Requirements The Trust also reserves the right to discuss the likelihood of a Potential Provider being successful in the procurement and reserves the right to de-select a Potential Provider should the other elements of their Proposal be of a significantly lesser quality compared to those submitted by other Potential Providers. 9.8 ARRANGEMENTS FOR SOLUTION DEMONSTRATIONS, SOLUTION VERIFICATION & REFERENCE SITE VISITS/CONFERENCE CALLS AND THE LINK TO BENEFITS 9.8.1 Introduction This section sets out the Trust’s current thinking surrounding requirements for Solution Demonstrations, Solution Verification and Reference Sites. 9.8.2 Solution Verification sessions and Solution Demonstrations Potential Provider Solution Verification sessions are likely to be required at the following stages in the procurement: At the Initial Tender stage. Those Potential Providers to be shortlisted to participate in the in the Initial Tender stage will be required to present a Solution Demonstration as part the presentation of their Initial Tenders During the Dialogue stage, in which the sessions are likely to be divided as follows or similar: o Patient Administration System functionality o Core clinical functionality o Pathology, Radiology, Cardiology, Pharmacy and other specialist departmental functionality o Technical functionality (for example user and access management, specialist form and content building, integration, interoperability and such like) o Clinical/Business Intelligence and Research platform functionality Commercially Confidential Version 1.1 Page 53 of 72 Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation For each of these areas the Trust will appoint a core representative team responsible for the scoring. Specialist sessions may additionally be run and scored to accommodate specialist functionality. Open Solution Demonstrations will form part of the Trust’s transformation and communications strategy designed to raise awareness of this Programme and of the potential solutions. The core representative teams may take informal soundings from these sessions to inform their thinking but the open sessions will not be scored in such a way as to significantly influence the scoring of the core teams. 9.8.3 Solution Verification 9.8.3.1 Introduction In parallel with the Dialogue stage, the Trust will be asking each shortlisted Potential Provider to work through a series of more detailed face to face “discovery” sessions where the Trust’s representatives will review Potential Provider demonstrations of Solution application functionality and, in some cases, the relating configuration which relates to key areas of core functionality. The Trust will provide scenarios and descriptions of the functionality and configuration that Potential Providers should demonstrate. This key Solution Verification activity will be crucial in identifying: The best fit Solution for the Trust The extent to which the demonstrated Solution functionality that has successfully delivered the benefits identified by the Trust (as well as other benefits proposed by Potential Providers) elsewhere is also likely to do in the Trust’s circumstances Where the track record reflected in the previous bullet does not exist, the extent to which the demonstrated Solution functionality is likely to successfully deliver the benefits identified by the Trust (as well as other benefits proposed by Potential Providers) in the Trust’s circumstances 9.8.3.2 Solution Verification Session Content and Specification Solution Verification sessions are anticipated to: Be based upon a scripted series of clinical questions or scenarios Potential Providers are expected to demonstrate system capability with the application, and where relevant, the system configuration/build tools These sessions will take place over a number of days and a timetable will be provided and agreed with all Potential Providers A sample Solution Verification session specification will be included in the version of this paper to be issued with the PQQ documentation. For each functional area, Solution Verification sessions will comprise of a set of individual scenarios or requirements. Unless the scenario is a request for information, the Trust will expect to see the functionality demonstrated by use of the application or configuration tool. Each scenario contains: Commercially Confidential Version 1.1 Page 54 of 72 Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation A description of the functionality to be demonstrated (the “question”) An example of the clinical scenario under which this would operate Example criteria which the Trust evaluators expect the functionality to meet Usability will be a critical factor in the assessment of functionality for each scenario. 9.8.3.3 Process and arrangements The intention is for the Solution Verification sessions to work through specified functional areas, in a prescribed order with each supplier. The Trust expects the code to be used in System Verification sessions to be the current or near-to-release code. Where a scenario can only be demonstrated by use of development or non-live code then this should be clearly stated by the supplier prior to the demonstration. Potential Providers will be asked for any specific requirements in advance of the sessions e.g. internet access etc. and the Trust will endeavour to meet reasonable requests and requirements in order to facilitate effective sessions. Potential Providers will be responsible for ensuring that their demonstration environment is functional and opportunity will be given prior to the sessions to test these. Those wishing to use remote environments are advised that the Trust cannot bear responsibility for any failure to access solutions beyond the Trust’s control e.g. internet access beyond the Trust’s firewalls, non-Trust-side power failures etc. Shortlisted Potential Providers will have the opportunity to discuss Solution Verification guidelines with the Trust in more detail following their issue as part of the ITPD. In addition, these days will provide the opportunity for experts from Potential Providers to discuss technical issues with Trust counterparts. Additional detail surrounding Solution Verification arrangements will be provided in the ISIT documentation. 9.8.4 Benefits Responses The benefits related starting point of the Dialogue stage will surround the Initial Tender responses to have been provided by shortlisted Potential Providers. The Trust will feedback to Potential Providers on the relative merits of their benefits proposals in for discussion with the Trust during an early initial benefits discussion with Potential Providers as part of the first Initial Dialogue session. That discussion will aim to agree the process for further developing Potential Provider benefits proposal in leading to Final Tenders. As part of that process: If necessary, Potential Providers will then be allowed further access to the Trust to hold benefits discussions with key stakeholders in parallel with the Further Dialogue sessions; o Potential Provider requests for additional information and/or access to the Trust as part of developing their benefits proposals surrounding, for example: The readiness of the Trust to deliver specified benefits Commercially Confidential Version 1.1 Page 55 of 72 Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation The willingness and ability of the Trust to undertake the specific actions that would be required to be undertaken in order to realise the specified benefits, or, to work with the Potential Provider on jointworking responsibilities The willingness of the Trust’s budget holders to sign up to benefits initiatives and commit to releasing savings Assessing the extent to which savings relate to budget items that are easy, or difficult, to extract from Trust’s budgets o However, the Trust will wish to discuss with Potential Provider specific ways that balance Potential Provider requests for any additional information and/or for any access to the Trust as part of developing their benefits proposals with the need to be mindful of the associated opportunity costs for all parties o The Trust will schedule Benefits Review Sessions with Potential Providers to discuss their Initial Tenders, the Trust response to them and next steps. Those sessions will be undertaken in parallel with the Dialogue sessions 9.8.5 Reference Site Visits/Conference Calls Reference Site Visits & Calls will also take place during the Dialogue Stage. Potential Providers are required to provide, as part of PQQ responses, a full list of sites using their Solution, together with details of whether they are specialist paediatric, specialist tertiary/quaternary or general hospitals, from which reference site will be chosen. From these, the Trust will, in discussion with Potential Providers, select a site or sites to be the subject of a reference site visit and further sites to be the focus of conference calls. The Potential Provider will be responsible for making arrangements with the sites concerned. The profiles of the sites that the Trust would ideally wish to see referenced in PQQ responses, and to be subject to reference sites visits are those that: Most closely match the size and nature of the Trust, including a clear paediatric focus Clearly demonstrate the highly effective use of a tried and tested PAS in circumstances that either: o fully satisfies the requirements of the Trust’s funding bodies or, failing that o very closely satisfies those requirements and would require very minimal development in order to conclusively do so Represent a site whose implementation of the solution is well executed, reducing the risk that solutions are degraded by implementation issues The Reference Site visits and teleconferences will be used to inform the confidence that the functionality is usable in the clinical setting, and inform the confidence assessment of the implementation delivery, of the benefits profiles and the risk of additional costs contributing Commercially Confidential Version 1.1 Page 56 of 72 Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation to the Total Cost of Ownership and Potential Providers will be expected to facilitate such discussions with reference sites. The dates and times of visits and calls will be allocated in discussion with Potential Providers and their Reference Sites. Definitive Reference Site guidelines, including agenda, and a standard question list to provide structure and consistency for visits, will be provided with the ITPD documentation (as will the equivalent for any conference calls that are undertaken). Please note that the Trust’s early thinking surrounding reference sites is that they are unlikely to be weighted highly in their own right, though the insights gained may be used to revisit the scoring of functionality if the visit sheds doubt on the usability of the functionality in live clinical practice. Reference site visit issues will also feed the Dialogue stage of the procurement and/or be assessed as part of other Final Tender evaluation criteria in terms of the extent to which the reference sites provide the Trust with delivery confidence and delivery assurance in confirming, enhancing, or calling into question aspects of Initial Tenders and/or emerging Final Tenders. 9.9 STAGE 4 - CONTRACT AWARD Following receipt of Potential Provider ISFT responses, evaluation will take place. Preparation of the Full Business Case (FBC) will take place in parallel with dialogue and with contract clarification. FBC approval and contract award will be subject to Trust Board approval. 9.10 FINAL TENDER ARRANGEMENTS The outline Final Tender evaluation criteria and weightings will be presented in a future version of this document. 9.11 PROCUREMENT SUPPORT FOR THE PROGRAMME Procurement support for the project will be provided by Howard Lewis, EPR Procurement Manager, supported by external advisers as appropriate. Commercially Confidential Version 1.1 Page 57 of 72 Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation 10. POTENTIAL PROVIDER BRIEFING EVENT 10.1 INTRODUCTION AND CONSULTATION POINTS The Trust is holding a Potential Provider Event on 21st and 22nd January 2015 in Central London This market engagement is designed to test the market in terms of the achievability of the scope, scale and timeframe of our requirements and to encourage input from Potential Providers. Of particular interest are the following consultation points: The capacity and capability of Potential Providers to deliver each aspect of the required EPR-enabled transformation programme The achievability of the Trust’s implementation timescales The achievability of the indicative procurement timetable, given the size and scope of the procurement project The likely level of Potential Provider interest in this exciting opportunity The extent to which the Trust might consider additions or variations to the proposed approach set out in this Paper that might make this a more attractive opportunity to Potential Providers The extent to which the Trust might provide additional information, or clarification, in order to inform Potential Provider decision making about whether to respond at the Pre-Qualification Questionnaire (PQQ) stage The extent to which the Trust might need to work on the provision of additional information, or clarification, in order to inform the contribution of shortlisted Potential Providers during the Dialogue stage The extent to which some of the Trust’s requirements could potentially be delivered via Lot 1 and, also, via Lot 2 and, if so, how such a scenario would impact on the procurement The Trust very much hopes that this open and consultative market engagement approach will enable them to get the design of the Dialogue stage of the procurement right by testing, in an informal setting, the likely market response to this opportunity. The Trust also hopes that by starting the process relatively early in this informal manner, Potential Providers will be better placed to respond in an effective manner to our PQQ than would otherwise be the case. A draft Potential Provider Briefing Event Agenda is presented at Appendix 5. Details of the Trust Procurement Team who are expected to be at the event are provided at Appendix 6. Commercially Confidential Version 1.1 Page 58 of 72 Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation 10.2 ONE TO ONE EPR REQUIREMENT DISCUSSIONS There will be the opportunity for Potential Providers to request a 55 minute, one to one discussion with key personnel from the Trust on 21st January 2016. Potential Providers are invited to apply, via https://www.lppsourcing.org/procontract/lpp/supplier.nsf/, for one of slots listed below using the proforma provided at Appendix 2. If demand for these slots exceeds the time available on the day, the Trust will also make available additional teleconference slots. However, please note that the Trust is keen to ensure that both Potential Provider and Trust time is used to good effect. The Trust therefore reserves the right to confine the use of these one to one slots to those Potential Providers able to demonstrate experience of successfully delivering similar EPR requirements in healthcare organisations of a similar size and nature to those required under this Contract, as specified in Appendix 2. Slot 1: 11.00 - 11.55 Slot 2: 12.00 - 12.55 Slot 3: 13.30 - 14.25 Slot 4: 14.30 - 15.25 Slot 5: 15.45 - 16.35 Slot 6: 16:40 – 17.35 Slot 7: 17.50 – 18.45 10.3 ONE TO ONE CLINICAL / BUSINESS INTELLIGENCE AND RESEARCH PLATFORM REQUIREMENT DISCUSSIONS There will be the opportunity for Potential Providers to request a 55 minute, one to one discussion with key personnel from the Trust on 22nd January 2016. Potential Providers are invited to apply, via https://www.lppsourcing.org/procontract/lpp/supplier.nsf/, for one of slots listed below using the proforma provided at Appendix 3. If demand for these slots exceeds the time available on the day, the Trust will also make available additional teleconference slots. However, please note that the Trust is keen to ensure that both Potential Provider and Trust time is used to good effect. The Trust therefore reserves the right to confine the use of these one to one slots to those Potential Providers able to demonstrate experience of successfully delivering similar Clinical / Business Intelligence and Research Platform requirements in healthcare organisations of a similar size and nature to those required under this Contract, as specified in Appendix 3. Slot 1: 11.00 – 11.55 Slot 2: 12.00 – 12.55 Slot 3: 13.30 – 14.25 Slot 4: 14.30 – 15.25 Commercially Confidential Version 1.1 Page 59 of 72 Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation Slot 5: 15.45 – 16.40 Slot 6: 16.45 – 17.40 10.4 COMMUNICATING THE ISSUES ARISING FROM THE BRIEFING EVENT The Trust will be adopting an equitable approach to all that it does and will therefore incorporate, in an appropriate manner, all substantive issues that arise in our project and procurement documentation. Our next step will be to issue a Frequently Asked Questions (FAQ) document containing Questions and Answers that arise in advance of, during and subsequent to the Potential Provider Event. These FAQs will be reflected as appropriate in subsequent documentation. Consequently, unless clearly and specifically stated, any information you provide at the event and during one to one Potential Provider discussions will be considered to be nonconfidential. Should you wish to share information that you would like the Trust to keep confidential because of commercial sensitivity, please make this clear to us before disclosing by marking it “Not for disclosure to third parties"; we can then discuss further. The Trust does not accept any duty of confidence because any information is designated that it is not to be disclosed to third parties where in the Trust’s opinion the information is disclosable under the Freedom of information Act 2000 (or Environmental Information Regulations 2004) and there are no grounds for any exemption to disclosure. [End of main body of the document] Commercially Confidential Version 1.1 Page 60 of 72 Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation 11. APPENDICES APPENDIX 1. TRUST REQUEST FOR INDICATIVE POTENTIAL PROVIDER PRICING 1. THE TRUST Activity for the Trust in 2014/2015 was as follows: Activity Type Figures Surgical Figures Theatres (includes procedure rooms) 27 Inpatient operations 60% of total Day case operations 40% of total Total elective & emergency operations (includes procedures 19,650 such as MRI/IR) Outpatient Figures Outpatient Visits (excludes multiple on same day) 242,140 Inpatient Figures Staffed Beds Inpatient Days Day Cases Admissions 363 94,066 25,354 40,785 Specialty Visit/ Procedure Figures Oncology Ophthalmology Radiology Cardiology Laboratory Figures Pathology Investigations Anatomic Pathology Commercially Confidential Version 1.1 7,600 6,682 55,000 23,307 969,073 5,754 Page 61 of 72 Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation Potential Providers are requested to make their own assessment of peak concurrency levels using the Trust staff numbers provided. We have 3,874 staff including: • • • • • • • • 585 Medical and Dental 1,289 Nurses 739 in administrative and managerial roles 198 Allied Health Professionals 258 Healthcare scientists 453 in Clinical Support and Technical services 236 Professional Scientific staff 113 Estates and Ancillary staff The principal clinical divisions and departments of the Trust are as follows: Medical Division Adolescent Medicine Endocrinology Gastroenterology Pharmacy Clinical Genetics Metabolic Medicine Nephrology Neurosciences Division Child and Adolescent Mental Health Services Neurodisability Neurology Neuropsychology Neurosurgery Ophthalmology Clinical Neurophysiology Clinical Site Practitioners Bed Management ICI Division Bone Marrow Transplant Dermatology Haematology Infectious Diseases Oncology Immunology Commercially Confidential Version 1.1 Page 62 of 72 Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation Palliative Care Rheumatology Cardio-respiratory & Critical Care Division Cardiac Surgery Cardiology Cardiothoracic Transplantation CICU PICU/NICU Pulmonary Hypertension Respiratory Sleep Children's Acute Transport Service Diagnostic services Nuclear Medicine Angiography Bone Density Radiology Diagnostic Imaging Fluoroscopy Magnetic Resonance Imaging Main X-Ray Ultrasound ECG ECMO Cardiology MRI Cardiopulmonary Exercise Laboratory Clinical Echocardiography Laboratory services Blood Transfusion Chemical Pathology Blood Sciences Microbiology Molecular Genetics Histopathology Virology Haematology Histopathology Cytogenetics Microbiology Commercially Confidential Version 1.1 Page 63 of 72 Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation Immunology Allied Health Professional services Psychological Medicine Dietetics Occupational Therapy Physiotherapy Speech and Language Therapy Play Therapy Psycho-Social & Family Services Surgical departments 2. Anaesthetics Audiological Medicine Cleft Cochlear Implant Craniofacial Dental and Maxillofacial Surgery Ear Nose and Throat Orthopaedic Surgery Spinal Surgery Pain Management Urology Plastic Surgery THE SCOPE “Core” requirements The indicative pricing for the EPR solution should reflect the core functions outlined in section 0, giving an indication of sub-contractor pricing where the Potential Provider intends to propose such an arrangement. If the supplier’s solution does not meet the requirement and no subcontracting arrangement is likely to be proposed (i.e. the Potential Provider does not have a proposed solution for a functional area and the Trust will be expected to make other arrangements), this should be indicated clearly. Optional elements The Trust has also identified a number of optional elements to supplement the core solutions that are also detailed within section 0. Indicative pricing should be provided where the Potential Provider intends to propose a solution to meet these optional requirements. Commercially Confidential Version 1.1 Page 64 of 72 Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation 3. INDICATIVE PRICING ASSUMPTIONS Please assume the following in the provision of your indicative pricing: 4. The Trust sizing volumes provided in the section ‘The Trust’ above The functional and non-functional requirements and contract duration summarised in ‘The Scope’ above All prices should be exclusive of VAT, other taxes & indexation INDICATIVE PRICING Please provide your indicative pricing using the tables below. If there is a price for a module which covers more than one component of the functionality below that you cannot extract you should make this clear in your indicative pricing. Ref EPR Components 1 People and Teams 2 Care Pathways and Decision Support 3 Patient Management - Referral 4 Patient Management – Out Patients 5 Patient Management – In Patients 6 Theatres and Surgery 7 Protecting the Child 8 Record-keeping 9 Information Presentation 10 Requesting and Fulfilment - General 11 Requesting and Fulfilment - Drugs 12 Requesting and Fulfilment - Pathology 13 Requesting and Fulfilment – Imaging 14 Resource Management 15 Leaving GOSH 16 Specialty-specific requirements within the EPR Commercially Confidential Version 1.1 Total OneOff Charges (Software & Professional Services) Annual Recurring Charges (Licence Support & Maintenance) Page 65 of 72 Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation Ref EPR Components 17 18 19 20 21 Clinical and Business Intelligence Communications and Collaboration Master Data Management Research and Trials Platform Commercially Confidential Version 1.1 Total OneOff Charges (Software & Professional Services) Annual Recurring Charges (Licence Support & Maintenance) Page 66 of 72 Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation APPENDIX 2. TRUST PROFORMA TO REQUEST A ONE TO ONE MEETING TO DISCUSS EPR REQUIREMENTS 1.1 Company Details. Legal name and website of the EPR Potential Provider (sometimes referred to as ‘suppliers’ or ‘bidders’) in whose name the potential tender will be submitted (the Prime or Single contractor): Company Name Website Name of any proposed or optional sub-contractors and the functional area for which the subcontractors are proposed Website of any proposed sub-contractors 1.2 Contact Details. Name, position, telephone number and e-mail address of main one to one meeting contact (please note that any absence of the prime contact should be notified to the Trust with details of the replacement contact): Name Position Telephone number Mobile telephone number E-mail address 2.1. EPR Referencability. Please summarise briefly below, in no more than 500 words, your experience of successfully delivering similar EPR requirements in healthcare organisations of a similar size and nature to those required under this Contract. RESPONSE: Commercially Confidential Version 1.1 Page 67 of 72 Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation APPENDIX 3. TRUST PROFORMA TO REQUEST A ONE TO ONE MEETING TO DISCUSS CLINICAL / BUSINESS INTELLIGENCE AND RESEARCH PLATFORM REQUIREMENTS 1.1 Company Details. Legal name and website of the Clinical / Business Intelligence and Research Platform Potential Provider (sometimes referred to as ‘suppliers’ or ‘bidders’) in whose name the potential tender will be submitted (the Prime or Single contractor): Company Name Website Name of any proposed or optional sub-contractors and the functional area for which the subcontractors are proposed Website of any proposed sub-contractors 1.2 Contact Details. Name, position, telephone number and e-mail address of main one to one meeting contact (please note that any absence of the prime contact should be notified to the Trust with details of the replacement contact): Name Position Telephone number Mobile telephone number E-mail address 2.1. Clinical / Business Intelligence and Research Platform Referencability. Please summarise briefly below, in no more than 500 words, your experience of successfully delivering similar Clinical / Business Intelligence and Research Platform requirements in healthcare organisations of a similar size and nature to those required under this Contract. RESPONSE: Commercially Confidential Version 1.1 Page 68 of 72 Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation APPENDIX 4. CURRENT GOSH CORE CLINICAL SYSTEMS The table below displays the main systems in use at the Trust. Current Core Clinical Systems Activity Recording Tool Bespoke system which allows support staff such as AHPs, Clinical Nurse Specialists capture time spent with patients. Activity can be recorded by PC or mobile device. To capture patient and non-patient related tasks in order to support business case. Auditbase Electronic Information System in use in the GOSH Audiology department for the documentation of assessments, test results, treatment plans, letter generation and hearing aid stock management. Auditbase links with specialist external hardware used to conduct hearing aids. Its install also provides access to the industry wide database of hearing hardware known as NOAH. CareVue Phillips Electronic Information System in use in the ICUs and HDU for capturing structured multidisciplinary notes and observations. The system is integrated with blood gas machines and vital signs monitors and handles prescribing within ICU only. Bespoke Multidisciplinary system for Craniofacial Patients. Allows information and images to be gathered and shared between members of the large multidisciplinary team involved in caring for craniofacial patients. JAC Electronic Prescribing, Medicines Administration and Pharmacy stock control. Used in all inpatient areas with the exception of ICU. Not currently used in outpatients. Craniofacial Patient Platform EPMA i.PM Nervecentre OMNI-Lab CSC (formerly iSOFT) Patient Administration System, including standard PAS functions (Master Patient Index, outpatient appointments, inpatient management), plus theatre scheduling and order entry for radiology and pathology (N.B. not electronic order communications or results reporting). Electronic Observations programmed with GOSH-developed EWS (Children’s’ Early Warning System, CEWS), Automated alerting (sent by way of message over Wi-Fi to the recipient), Task Management and instant messaging. Also used for hospital at night handover. In use in all inpatient wards with the exception of Critical Care and HDU wards. System is accessed largely via Apple mobile devices. Laboratory Information Management System supplied by Integrated Software Solutions. Used by all the main Pathology disciplines as well as for Regional Genetics and Newborn Screening laboratories. Commercially Confidential Version 1.1 Page 69 of 72 Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation Current Core Clinical Systems PACS GE Centricity PACS 4.0. Electronic storage and workflow for DICOM Images within the Trust, allowing trust users to view history of patient images to aid decisions for on-going care, management as well as research and teaching purposes. PANDA Tool used to objectively measure paediatric dependency and acuity for patients in acute inpatient services in order to predict the staffing requirements for wards as part of the workforce planning process. The tool is also used to provide information on the categories of dependency and acuity of patients on each ward to shape training and education programmes. Originally a paper toolkit, developed by GOSH, PANDA is in place in several other paediatric sites. GOSH retain the intellectual property rights to this tool. Photo ID Bespoke system supporting the use of images for patient identifying patients who are unable to wear a wrist band. Photo taking and identity checking functionality. Manages lifecycle of photographs. PSAG Patient Status at a Glance. In-house developed electronic whiteboard of inpatient status. Currently being rolled out across all inpatient wards. RIS GE Centricity RIS v 5.0.9. System to provide workflow for scheduling, recording of Radiology examinations within the trust, integrates with PACS System to provide imaging workflow for Radiology Reporting. TomCat Philips CVIS Cardio-Vascular Information system, (previously known as Tomcat). Information system for booking and reporting cardiac procedures and booking and recording outcomes of MDT meetings. Vital Pulse Renal pathway system including dialysis machine connectivity and transplant. Produces returns for UK renal registry. Commercially Confidential Version 1.1 Page 70 of 72 Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation APPENDIX 5. POTENTIAL PROVIDER BRIEFING EVENT AGENDA We reserve the right to amend the dates and timings of this agenda EPR ENABLED TRANSFORMATION PROCUREMENT Potential Provider Briefing Day-EPR st 21 January, Weston House Lecture Theatre, Level 2 Weston House, Great Ormond Street, WC1N 3HZ Agenda 08.30 – 09.00 Registration with refreshments - 09.00 – 10.00 Trust Presentations – Weston House Lecture Theatre Welcome & Introduction to the opportunity Overview of the Transformation Programme Rationale for the Programme Overview of the Procurement Process 10:00– 10:45 Open Forum / Q&A Session – Weston House Lecture Theatre Open forum for Potential Provider questions and clarification. 11:00 – 17:35 One to one EPR Requirement Session Slots with Potential Providers. The Trust will make seven 55 minute one to one Potential Provider Slots available, to be booked on a first come first served basis. The slots available are as follows: Slot 1: 11.00 - 11.55 Slot 2: 12.00 - 12.55 Slot 3: 13.30 - 14.25 Slot 4: 14.30 - 15.25 Slot 5: 15.45 - 16.35 Slot 6: 16:40 – 17.35 Slot 7: 17.50 – 18.45 EPR ENABLED TRANSFORMATION PROCUREMENT Commercially Confidential Version 1.1 Page 71 of 72 Potential Provider Briefing Document and Descriptive Document for Pre-Procurement Market Consultation Potential Provider Briefing Day-Clinical/Business Intelligence and Research 22nd January, Location TBC Agenda 09.00 – 09.45 Trust Presentations – Location to be confirmed Welcome & Introduction to the opportunity Overview of the Research and the Transformation Programme Overview of the Procurement Process 09.45– 10:30 Open Forum / Q&A Session Location tbc Open forum for Potential Provider questions and clarification. 11:00 – 17.40 One to one Clinical / Business Intelligence and Research Platform requirement Session Slots with Potential Providers – Location tbc The Trust will make six 55 minute one to one Potential Provider Slots available, to be booked on a first come first served basis. The slots available are as follows: Slot 1: 11.00 – 11.55 Slot 2: 12.00 – 12.55 Slot 3: 13.30 – 14.25 Slot 4: 14.30 – 15.25 Slot 5: 15.45 – 16.40 Slot 6: 16.45 – 17.40 APPENDIX 6. TRUST PROCUREMENT TEAM AND CONTACT DETAILS The procurement project contract address to be used throughout the procurement is: https://www.lppsourcing.org/procontract/lpp/supplier.nsf/ Commercially Confidential Version 1.1 Page 72 of 72