Appendix. - BioMed Central
advertisement
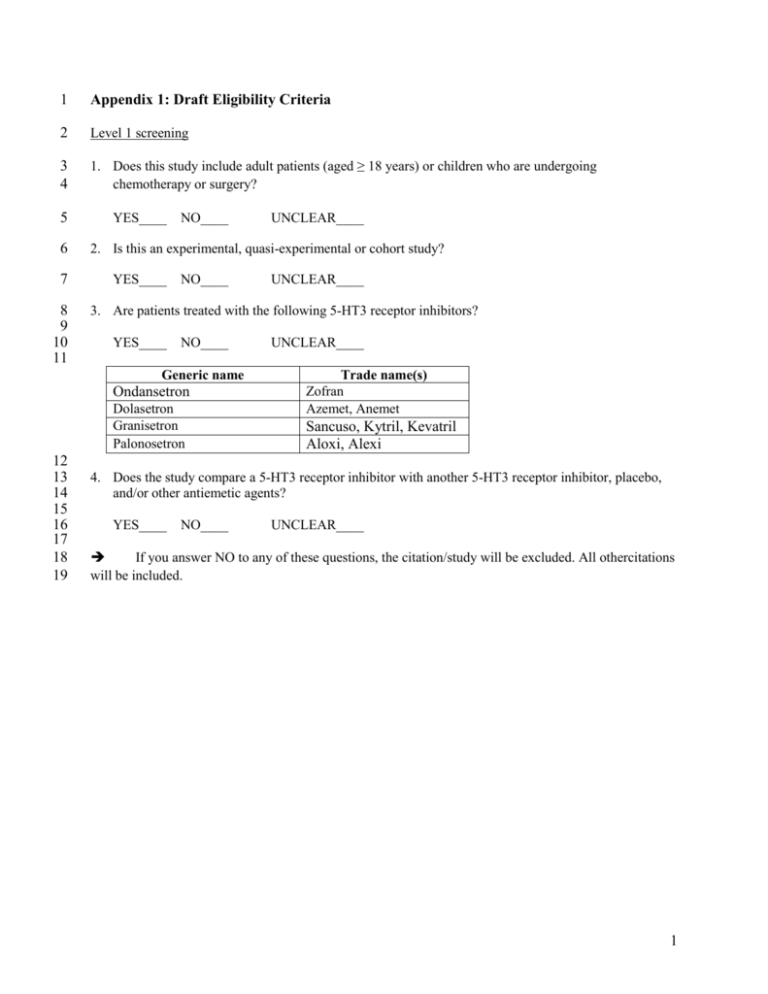
1 Appendix 1: Draft Eligibility Criteria 2 Level 1 screening 3 4 1. Does this study include adult patients (aged ≥ 18 years) or children who are undergoing chemotherapy or surgery? 5 6 7 8 9 10 11 YES____ NO____ 2. Is this an experimental, quasi-experimental or cohort study? YES____ NO____ UNCLEAR____ 3. Are patients treated with the following 5-HT3 receptor inhibitors? YES____ NO____ Generic name Ondansetron Dolasetron Granisetron Palonosetron 12 13 14 15 16 17 18 19 UNCLEAR____ UNCLEAR____ Trade name(s) Zofran Azemet, Anemet Sancuso, Kytril, Kevatril Aloxi, Alexi 4. Does the study compare a 5-HT3 receptor inhibitor with another 5-HT3 receptor inhibitor, placebo, and/or other antiemetic agents? YES____ NO____ UNCLEAR____ If you answer NO to any of these questions, the citation/study will be excluded. All othercitations will be included. 1 20 Level 2 screening 21 22 1. Does this study include adult patients (aged ≥ 18 years) or children who are undergoing chemotherapy or surgery? 23 24 25 26 27 28 29 YES____ NO____ UNCLEAR____ 2. Is this an experimental, quasi-experimental or cohort study? YES____ NO____ UNCLEAR____ 3. Are patients treated with the following 5-HT3 receptor inhibitors? YES____ NO____ UNCLEAR____ Generic name Trade name(s) Zofran Azemet, Anemet Ondansetron Dolasetron Granisetron Palonosetron 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 Sancuso, Kytril, Kevatril Aloxi, Alexi 4. Does the study compare a 5-HT3 receptor inhibitor with another 5-HT3 receptor inhibitor, placebo, and/or other antiemetic agents? YES____ NO____ UNCLEAR____ 5. Does the study report at least one of the following outcomes? Arrhythmia, sudden cardiac death, QT prolongation, PR prolongation, all-cause mortality, nausea, or vomiting. YES____ NO____ UNCLEAR____ If you answer NO to any of these questions, the citation/study will be excluded. All other full-text articles will be included. 2 46 Appendix 2: Draft literature search for MEDLINE 47 48 49 50 51 52 53 54 55 56 57 58 59 60 61 62 63 64 65 66 67 68 69 70 71 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 87 88 89 90 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 Ondansetron/ [ Ondansetron ] ondansetron.mp. zofran.mp. SN-307.mp. SN307.mp. GR38032F.mp. GR-38032F.mp. GR C50775.mp. 99614-02-5.rn. [ CAS Registry ] bryterol.mp. cedantron.mp. ceramos.mp. emeset.mp. modifical.mp. narfoz.mp. onsia.mp. sakisozin.mp. vomceran.mp. zofrene.mp. zefron.mp. zophron.mp. zophran.mp. zuplenz.mp. zophren.mp. zudan.mp. Granisetron/ [ Granisetron ] granisetron$.mp. kytril.mp. BRL-43694.mp. BRL43694.mp. 109889-09-0.rn. [ CAS Registry ] apf 530.mp. eutrom.mp. granicip.mp. granisol.mp. kevatril.mp. sancuso.mp. taraz.mp. dolasetron.mp. [ Dolasetron ] anzemet.mp. anemet.mp. zamanon.mp. MDL 73,147EF.mp. MDL-73147EF.mp. 3 91 92 93 94 95 96 97 98 99 100 101 102 103 104 105 106 107 108 109 110 111 112 113 114 115 116 117 118 119 120 121 122 123 124 125 126 127 128 129 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 61 62 63 64 65 66 67 68 69 70 71 72 73 74 75 76 77 78 79 80 81 dolasetron.rn. [ CAS Registry ] palonosetron.mp. 135729-61-2.rn. [ CAS Registry ] onicit.mp. aloxi.mp. 2-Qhbiqo.mp. Serotonin 5-HT3 Receptor Antagonists/ 5ht3.mp. 5-HT3.mp. "5-Hydroxytryptamine-3 receptor antagonist?".mp. "serotonin type 3 receptor antagonist$".mp. "5-hydroxytryptamine-3 antagonist$".mp. or/1-56 chemotherap$.mp. [ chemotherapy ] chemo-therap$.mp. Antineoplastic Combined Chemotherapy Protocols/ exp Antineoplastic Agents/ canc$.mp. [ cancer / oncology ] carcinoma$.mp. tumo?r$.mp. neoplasm$.mp. on?olog$.mp. surger$.mp. [ surgery ] surgical$.mp. su.fs. exp Surgical Procedures, Operative/ Nausea/ [ nausea and vomiting ] Vomiting/ nause$.mp. vomit$.mp. emesis.mp. PONV.mp. "Postoperative Nausea and Vomiting"/ or/58-77 57 and 78 exp Animals/ not (exp Animals/ and Humans/) 79 not 80 4