Appendix: Draft Eligibility Criteria Level 1 screening Does this study
advertisement

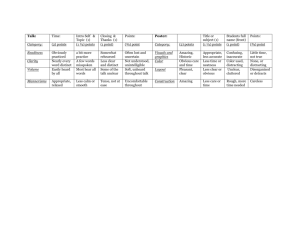
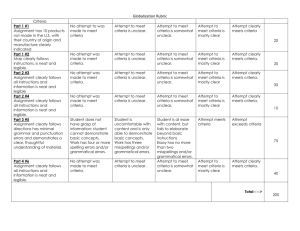
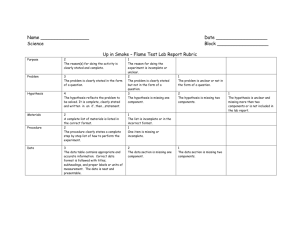
1 Appendix: Draft Eligibility Criteria 2 Level 1 screening 3 4 1. Does this study include adult patients (aged ≥ 18 years) or children who are undergoing chemotherapy or surgery? 5 6 7 8 9 10 11 YES____ NO____ 2. Is this an experimental, quasi-experimental or cohort study? YES____ NO____ YES____ NO____ Ondansetron Dolasetron Granisetron Palonosetron 22 23 UNCLEAR____ 3. Are patients treated with the following 5-HT3 receptor inhibitors? Generic name 12 13 14 15 16 17 18 19 20 21 UNCLEAR____ UNCLEAR____ Trade name(s) Zofran Azemet, Anemet Sancuso, Kytril, Kevatril Aloxi, Alexi 4. Does the study compare a 5-HT3 receptor inhibitor with placebo or supportive care? YES____ NO____ UNCLEAR____ 5. Does the study examine the use of interventions to mitigate cardiac risk (e.g., telemetry, ECG monitoring, adjustment of antiarrhythmics, electrolyte monitoring and replacement)? YES____ NO____ UNCLEAR____ If you answer NO to any of these questions, the citation/study will be excluded. All othercitations will be included. 1 24 Level 2 screening 25 26 1. Does this study include adult patients (aged ≥ 18 years) or children who are undergoing chemotherapy or surgery? 27 28 29 30 31 32 33 YES____ NO____ UNCLEAR____ 2. Is this an experimental, quasi-experimental or cohort study? YES____ NO____ UNCLEAR____ 3. Are patients treated with the following 5-HT3 receptor inhibitors? YES____ NO____ UNCLEAR____ Generic name Trade name(s) Zofran Azemet, Anemet Ondansetron Dolasetron Granisetron Palonosetron 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 Sancuso, Kytril, Kevatril Aloxi, Alexi 4. Does the study compare a 5-HT3 receptor inhibitor with placebo or supportive care? YES____ NO____ UNCLEAR____ 5. Does the study examine the use of interventions to mitigate cardiac risk (e.g., telemetry, ECG monitoring, adjustment of antiarrhythmics, electrolyte monitoring and replacement)? YES____ NO____ UNCLEAR____ 6. Does the study report at least one of the following outcomes? Arrhythmia, sudden cardiac death, QT prolongation, PR prolongation, all-cause mortality, nausea, or vomiting. YES____ NO____ UNCLEAR____ If you answer NO to any of these questions, the citation/study will be excluded. All other full-text articles will be included. 55 56 2