Gravimetric Analysis Lab
advertisement

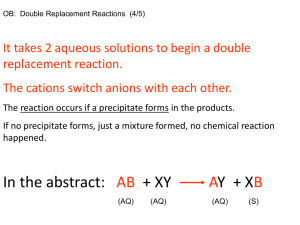
The Grav Lab (Gravimetric Analysis of an Unknown Chloride) Objective: To illustrate typical techniques used in gravimetric analysis by quantitatively determining the amount of chloride in an unknown. Background Information Quantitative analysis is that aspect of analytical chemistry concerned with determining how much of one or more constituents are present in a particular sample of material. Information such as percentage composition is essential to establishing formulas for compounds. Two common quantitative methods used in analytical chemistry are gravimetric and volumetric analysis. Gravimetric analysis derives its name from the fact that the constituent being determined can be isolated in some weighable form. Volumetric analysis, on the other hand, derives its name from the fact that the method used to determine the amount of a constituent involves measuring the volume of a reagent. Usually, gravimetric analyses involve the following steps: 1. Dissolving the samples. 2. Precipitating the constituent in the form of a substance of known composition by adding a suitable reagent. 3. Isolating the precipitate by filtration. 4. Washing the precipitate to free of contaminants. 5. Drying the precipitate to a constant mass (to obtain an analytically weighable form of known composition). 6 . Calculating the percentage of the desired constituent from the masses of the sample and precipitate. Although the techniques of gravimetric analysis are applicable to a large variety of substances, we have chosen to illustrate them with an analysis that incorporates a number of other techniques as well. Chloride ion may be quantitatively precipitated from solution by the addition of silver ion according to the following ionic equation: Ag+ (aq) + Cl-(aq) AgCl(s) Materials: Read the procedure and create the materials list from it. Equipment Chemicals Procedure: 1) Plug in hot plate and ensure that the setting is about 2/3 of the maximum setting. 2) Choose one of the unknowns, record its color, and mass LESS THAN 0.200 g directly into a clean and dry 150 mL beaker. 3) Dissolve the unknown chloride with 50.0 mL of 0.15 M HNO3. (The HNO3 will help prevent the precipitation of other anions with the silver ions) 4) Stir with a glass stirring rod. Keep the stirring rod in the solution to avoid losing chloride ions. 5) Place solution on the hot plate and let it heat up to about 50 ˚C without letting it boil. (The warm solution will cause the AgCl precipitate to coagulate, making it easier to filter) 6) While solution is warming, prepare the filtration flask and Buchner funnel. Be sure to mass the filter paper before using. 7) When solution is warm, add 50.0 mL of 0.10 M AgNO3 and stir. 8) When precipitate has formed then filter it out of the solution. 9) Use water bottle to ensure that all precipitate is out of the beaker. 10) Wash the precipitate with about 10 mL of dH2O then with two 5-7 mL portions of acetone. (Acetone helps remove some water) 11) Clean and weigh a watch glass. Label it with your name using a sharpie. 12) Carefully transfer the precipitate and the filter paper the watch glass and place it into the oven overnight. Grav Prelab Questions: 1) What would be a suitable alternative metal ion to precipitate the chloride? 2) A solution was made by dissolving 3.500 g of Strontium Chloride in 50.0 mL water. a. How many moles of Strontium Chloride are in the solution? b. How many moles of silver ion are needed to precipitate all of the chloride in the solution? c. If you are using 0.10 mol/L of silver nitrate, what volume would needed to be added to precipitate all of the chloride? 3) List 3 potential errors with that can occur with the experiment. Grav Prelab Questions: 1) What would be a suitable alternative metal ion to precipitate the chloride? 2) A solution was made by dissolving 3.500 g of Strontium Chloride in 50.0 mL water. a. How many moles of Strontium Chloride are in the solution? b. How many moles of silver ion are needed to precipitate all of the chloride in the solution? c. If you are using 0.10 mol/L of silver nitrate, what volume would needed to be added to precipitate all of the chloride? 3) List 3 potential errors with that can occur with the experiment.