Worksheet - Studentstt
advertisement

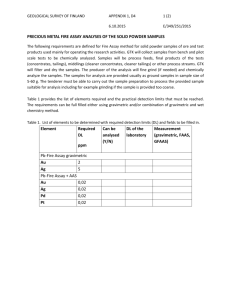
U2 mod 2chem. Gravimetric analyses 1 of 4 Gravimetric analysis describes a set of methods in analytical chemistry for the quantitative determination of an analyte based on the mass of a solid. In most cases, the analyte must first be converted to a solid by precipitation with an appropriate reagent. The precipitate can then be collected by filtration, washed, dried to remove traces of moisture from the solution, and weighed. The amount of analyte in the original sample can then be calculated from the mass of the precipitate and its chemical composition. When the precipitating reagent is added, there is a period of induction (time taken before the first sign of precipitate is seen) and then nucleation (when a minimum amount of ions come together to form a stable solid) occurs. Large crystals are preferred to small crystals as they are more easily filtered, digestion (heating of the solid and mother liquor [liquid containing the solid]) encourages the formation of large crystals. For filtration, a vacuum pump is used (large conical flask connected to a tap via an aspirator), sintered crucible are used (small crucible with very high melting point, inert) which can withstand high pressures used in vacuum filtration. A rubber policeman (glass rod with a hard rubber scrapper on one end) is also used to transfer residues of solid or precipitate onto glass surfaces. The precipitate is rinsed with cold distilled water and then heated in an oven to remove all traces of water and then cooled in a dessicator (air tight container which contains silica to absorb any water vapour present) before weighing. In other cases, the volitilisation method would be used. For example with a hydrated salt, the aim is to determine the number of moles of water of crystallisation present. It would be heated multiple times to remove the water ( and the difference in mass can then be used to determine the amount of water present and thus the number of moles of water needed for one mole of the anhydrous salt. NB Sometimes the element being analysed is in one form, but must first be converted to a different form before quantitative analysis can take place. Usually an acid would be used to convert the analyte which is a solid to an aqueous solution which can then be precipitated by an known amount of precipitating reagent. Either HCl or HNO3 is used, if the substance is a carbonate, the acid can be dilute, otherwise the concentrated version of the acid is used. Gravimetric method of calibrating a pipette The mass of a small empty beaker is measured and recorded using an analytical balance. The temperature of the water used is measured and recorded. Then using 10ml pipette, the liquid is drawn up and run into the small beaker and the mass of the beaker is measured and recorded. The previous step is repeated until there are 5 mass readings with water present. The relationship between density of water and its temperature is well known and the temperature recorded is used to obtain the density of the water used. The mean mass of the water delivered by the pipette is converted to volume using the relationship of density with mass and volume. The standard deviation of the data is calculated. U2 mod 2chem. Gravimetric analyses 2 of 4 Thus the mean volume and the standard deviation comprises the capacity of the pipette used. Checkpoint A 1. State briefly what steps one would take to determine the amount of water in a sample of hydrated magnesium sulphate crystals …………………………………………………………………………………… …………………………………………………………………………………… …………………………………………………………………………………… …………………………………………………………………………………… …………………………………………………………………………………… …………………………………………………………………………………… 2. Imagine you are given a sample of sea water and asked to determine the amount of chloride ions present. You decide to use the precipitation method, suggest a suitable reagent to use to form the precipitate………………………. Diagram of SOME apparatus used in gravimetric analysis The sintered glass filter can withstand the high pressures used in vacuum filtration. U2 mod 2chem. Gravimetric analyses 3 of 4 Uses of gravimetric analysis in quality control Measurement of the “essential” elements in plant foods (phosphorus, for example, is converted into the insoluble salt, magnesium ammonium phosphate) Estimation of pollutants in the air, such as sulphur dioxide (by conversion to insoluble barium sulphate) Estimation of sulphur dioxide (used to prevent microbial spoilage) in soft drinks, such as orange juice. Estimation of chloride ions in water supplies (by conversion to insoluble silver chloride). Worked Example of gravimetric analysis question A compound of Iron (Fe) and Chlorine (Cl) is soluble in water. An excess of Silver Nitrate (AgNO3) was added to precipitate the chloride ion as silver chloride. If a 134.8 mg sample of the compound gave 304.8 mg of AgCl, what is the formula of the compound? Answer: Note 1000 mg = 1g Ar Fe = 56, Cl = 35.5 Ag = 108 determine # mol AgCl = mass AgCl / molar mass AgCl = 0.3048 / (143.5) = 0.0021 Ratio AgCl : Cl- = 1: 1 therefore # mol Cl- = 0.0021 Mass of Cl- = # mol x relative atomic mass of Cl = 0.0021 x 35.5 = 0.0746g Mass of iron ions = mass of sample – mass of Cl- ions = 0.1348 – 0.0746 = 0.0602 g # mol iron = mass of iron / relative atomic mass iron = 0.0602 / 56 = 0.0011 Ratio of mol of iron : chloride = 0.0011 : 0.0021 1: 2 Therefore formula of compound = FeCl2 U2 mod 2chem. Gravimetric analyses 4 of 4 Checkpoint B 1. A 2.00g sample of limestone was dissolved in dilute hydrochloric acid and all the calcium present in the sample was converted to Ca2+(aq). Excess ammonium oxalate solution, (NH4)2C2O4(aq), was added to the solution to precipitate the calcium ions as calcium oxalate, CaC2O4(s). The precipitate was filtered, dried and weighed to a constant mass of 2.43g. Determine the % calcium in the limestone sample. Note: Ca2+(aq) + C2O42-(aq) CaC2O4(s) Ca = 40, N = 14, C = 12, H = 1, O = 16, Ag = 108, Cl = 35.5, Fe = 56 2. Note the dry precipitate would have the formula CaC2O4 END OF GRAVIMETRIC ANALYSIS