Solute – Solvent Interactions
advertisement
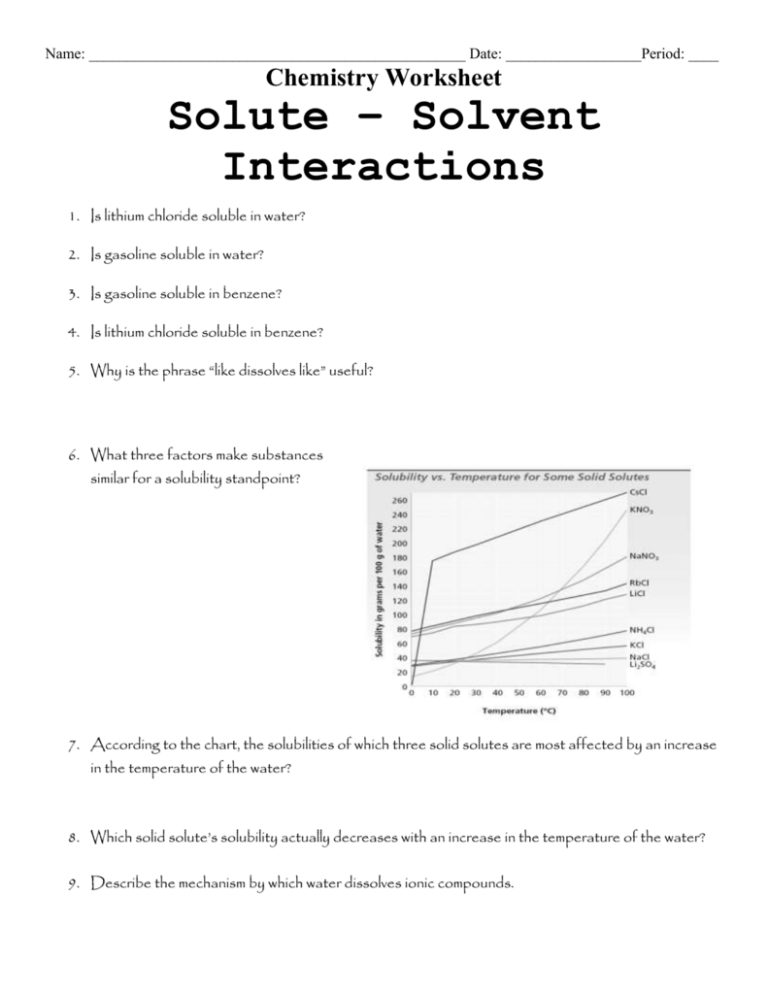
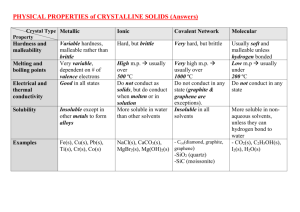
Name: __________________________________________________ Date: __________________Period: ____ Chemistry Worksheet Solute – Solvent Interactions 1. Is lithium chloride soluble in water? 2. Is gasoline soluble in water? 3. Is gasoline soluble in benzene? 4. Is lithium chloride soluble in benzene? 5. Why is the phrase “like dissolves like” useful? 6. What three factors make substances similar for a solubility standpoint? 7. According to the chart, the solubilities of which three solid solutes are most affected by an increase in the temperature of the water? 8. Which solid solute’s solubility actually decreases with an increase in the temperature of the water? 9. Describe the mechanism by which water dissolves ionic compounds. 10. Define the type of substance known as a “hydrate.” 11. How does heating affect a hydrate? 12. In general terms, what can be said about ionic solutes’ solubility in non-polar solvents? Explain. 13. Would you expect lithium chloride to dissolve in toluene? Why or why not? 14. Describe what happens when you shake a bottle of salad dressing. 15. Define the term “immiscible.” Give an example. 16. Define the term “miscible.” Give an example. 17. What effect does increasing pressure have on the solubilities of liquids or solids in liquid solvents? 18. What effect does increasing pressure have on the solubilities of gases in liquid solvents? 19. What effect does increasing temperature have on the solubilities of gases in liquid solvents? 20. What effect does increasing temperature normally have on the solubilities of solids in liquid solvents? 21. Are there exceptions to the factor discussed in Question 20? Explain.