Polarity and Solubility

Experiment #1 / Unit 7
Polarity and Solubility
Introduction:
Why do some substances mix and others do not? Why will water and oil remain two distinct layers? With an understanding of bonding and molecular structure we should be able to predict whether substances will interact with one another. The determining factor is whether a given molecule will have enough attraction for a "foreign" molecule to overcome any attractions to its own kind and allow them to mix. This can be decided by using the concept of polarity to determine what type of intermolecular attractions will be present. In this experiment, we will mix several different solvents and solutes and see which ones will dissolve and/or mix. Which solvents will be miscible? immiscible?
Which solvents will dissolve compounds made of ions, polar molecules, or nonpolar molecules?
Procedure:
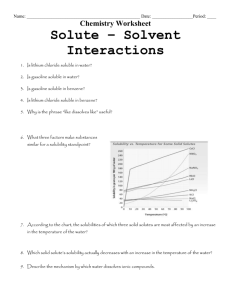
1. Obtain a microreaction plate, micro-test tubes, dropper bottles with solvents,
toothpicks, and cotton swabs.
2. Set up the tubes in the following fashion:
(1) water (2) ethanol (3) 2-propanone (4) 1-butanol (5) hexane
(a) water X
(b) ethanol X X
(c) KMnO
4
(d) CuCl
2
(e) iodine
3. Set up rows a-d: Add enough drops of solvents 1-5 to the tubes to allow you to observe the results.
Finish rows a + b: Add drops of water/ethanol to the appropriate tubes.
Stir with a toothpick and determine solubility.
Finish rows c + d: Add just a few crystals of each solute to the solvent in the test tubes. Try to add the same amounts to each tube.
Stir with a toothpick and determine if dissociation occurs and to what extent (using colored compounds will help you to determine how soluble they are in each solvent).
KMnO
4
will appear brown at low concentrations.
Row e: Take 5 test tubes to the hood and add one small crystal of iodine to each tube. Please do not drop any iodine anywhere because it will stain the floor (and your hands!) and the vapors are not healthy to breathe. Return to your lab station and add solvents 1-5. Stir with a toothpick and determine solubility. Iodine will appear yellow at low concentrations.
Chemistry II Cary Academy W.G. Rushin 1
4. Clean the test tubes thoroughly using the cotton swabs. A solution of 10%
Na
2
S
2
O
3
can be used to remove any I
2
stains because the thiosulfate ion
(S
2
O
3
2) is a good reducing agent and will reduce I
2
into colorless, water
soluble I
-
ions.
Data:
Record your observations in a copy of the above table. Use adjectives (very slightly) to differentiate between levels of solubility. Also differentiate between degrees of color (slightly purple).
Questions:
1. (a) Draw each solvent molecule (1-5).
(b) Determine the molecular polarity. For bipolar molecules, you can use terms like mostly polar (or nonpolar).
(c) List each type of attractive force present in the pure state for the molecule.
(d) Circle the force most responsible for the molecule's behavior.
2. Determine which of the solutes (a-e) are ionic or covalent. Determine
polarity and type of attractive forces for the molecules.
3. In a second table, describe the source of the attraction that allowed them to
mix or for those that were immiscible, briefly describe why they would not mix.
4. What does the rule, "Like dissolves/mixes with like", mean? Explain in terms
of attractions why it is true.
5. Explain the behavior of an ionic compound in the various solvents.
6. Explain the behavior of iodine in the various solvents.
7. Draw the structures for:
a. 2-butanone b. 1-hexanol c. 2-pentanol d. octane e. methanol
Lab Report #7.1:
title page
procedure sheet
data
questions
Chemistry II Cary Academy W.G. Rushin 2