373398_3_data_set_4091686_mkghyy
advertisement

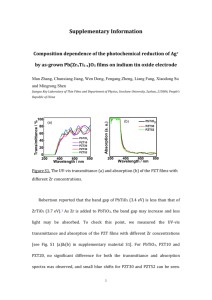
Supplementary Information Figure S1. (a) & (b) are reciprocal space mapping (RSM) scans on PZT/SRO/STO and PZT/LSCO/STO showing that fully c-oriented PZT has been attained in both heterostructure systems. Figure S2. High-resolution XRD performed on PZT/SRO/STO & PZT/LSCO/STO. The sole presence of (00l)-type peaks indicate that the films are fully c-oriented. Figure S3. (a) & (b) are histograms for Ag particle size distributions on PZT/SRO/STO and PZT/LSCO/STO, respectively. The median particle size on PZT/SRO/STO is 12% larger than the median particle size on PZT/LSCO/STO. S4. Numerical calculation for the temperature rise in SRO & LSCO electrodes cause by UV absorption. In order to estimate the heating caused by photon absorption at the PZT/electrode interface, first one needs to calculate the intensity profiles of the incident laser energy. Figure 1 compares the UV intensity profile as a function of penetration depth for the PZT/LSCO/STO (red) and PZT/SRO/STO (blue) heterostructures used in this paper. The UV intensity drop within each respective layer is calculated using1: I = (1-R)I0 e-αz (1) where I is the UV intensity at depth 𝑧, R is the reflectivity at an interface, I0 is the laser intensity at an interface before the occurrence of reflection and 𝛼 is the absorption coefficient. The absorption coefficient of PZT2 has been reported to be 3.8x105cm-1. The absorption coefficient for SRO is estimated as 1.2×105 cm-1 using the data provided in Koster et al.3, For LSCO we used the closest perovskite compound (CMR manganites)4 ~3.8×105cm-1. The reflectance values of PZT, SRO and LSCO and STO5 at 248 nm obtained from literature are given in Table 1. Figure S4. UV intensity profile as a function of penetration depth for the heterostructures used in this paper. To calculate the respective temperature rise cause by photon absorption at the electrode, we first calculated the heat diffusivity, D = k / Cp r 6 ,which governs the heat dissipation rate in a solid6. Following the model developed by Bharadwaja et al.,6 the characteristic heat diffusion length, (x) = (2Dτ)0.5, per laser shot is then determined. The temperature rise induced by each electrode can then be calculated using: DT = (1- R)I t C p r (2Dt )0.5 (2) where R, I, τ, Cp, ρ and D are the reflectivity, laser intensity, pules width (20ns), specific heat, material density and heat diffusivity, respectively. SRO LSCO Incident Laser Intensity (I0) on the PZT Layer 4.55 W/cm-2 4.55 W/cm-2 Laser Intensity (I1) Entering The PZT after reflection at air/PZT interface. The reflectance(R) of PZT is 19%6. 3.68 W/cm-2 3.68 W/cm-2 2.51 W/cm-2 2.51 W/cm-2 25% 7% I1= I0(1-RPZT) Incident Laser Intensity (I2) on Electrode (after PZT absorption) I2= I1 e-αz Where a is the absorption coefficient of PZT2 and z is the PZT thickness [10 nm] Reflectance7,8 (Relectrode) Laser Intensity (I3) Entering the Electrode (after reflection at PZT/electrode interface) 1.88x104 Wm-2 2.33x104 Wm-2 Theoretical Density of Electrode Material 9 6.489×103 kg m-3 6.55×103 kg m-3 Thermal Conductivity10,11 5.72 Wm-1 K-1 3.75 Wm-1 K-1 Heat Diffusivity 1.115×10-6 m2s-1 1.145×10-6 m2s-1 Specific Heat Capacitance(Cp )10,12 790J kg-1 K-1 449J kg-1 K-1 Characteristic Heat Diffusion Length (2Dτ)0.5 211nm 214nm ΔT (calculated from equation 2) 3.47×10-4 K 7.4×10-4 K I3= I2(1-Relectrode) D = k / Cp r Table 1. Calculations of the temperature rise within each respective electrode. It is found that the temperature rise caused by both electrodes is negligible. This is primarily because the majority of the laser energy is absorbed by the PZT layer. Moreover, both electrode materials have similar optical and thermal parameters. In conclusion, the thermal contribution from the electrodes is unlikely to be the cause of the observed difference in reaction rate of Ag+ ion reduction. References 1 2 3 4 5 6 7 8 9 10 11 12 P. Baeri, S. U. Campisano, G. Foti, and E. Rimini, J. Appl. Phys 50 (2) (1979). L. Tsakalakos and T. Sands, Appl. Phys. Lett. 76 (2), 227 (1999). G. Koster, L. Klein, W. Siemons, G. Rijnders, J. S. Dodge, C. B. Eom, D. H. A. Blank, and M. R. Beasley, Review of Modern Physics 84 (2012). J. H. Hao, X. T. Zeng, and H. K. Wong, J. Appl. Phys 79 (3), 3 (1996). S. J. Kim, S. S. A. Sea, S. J. Moon, and W. S. Choi, J. of the Korean Phys. Soc. 51, 5 (2007). S. S. N. Bharadwaja, T. Dechakupt, and S. T. McKinstry, J. Am. Ceram. Soc. 91 (5), 1580 (2008). H.S. Choi, J. Bak, J. S. Ahn, N. J. Hur, and T. W. Noh, J. of the Korean Phys. Soc. 33 (2), 184 (1998). Z. G. Hu, W. W. Li, Y. W. Li, M. Zhu, Z. Q. Zhu, and J. H. Chu, Appl. Phys. Lett. 94 (22) (2009). S. S. Vagarali and J. W. Lucek, United States (2004). S. Yamanaka, T. Maekawa, H. Muta, T. Matsuda, S. I. Kobayashi, and K. Kurosaki, J. Solid State Chem 177, 6 (2004). J. Androulakis, P. Migiakis, and J. Giapintzakis, Appl. Phys. Lett. 84 (7), 3 (2004). C. He, S. Eisenberg, C. Jan, H. Zheng, J. F. Mitchell, and C. Leighton, Phys. Rev. B 80 (21) (2009).