Supplementary Information
advertisement
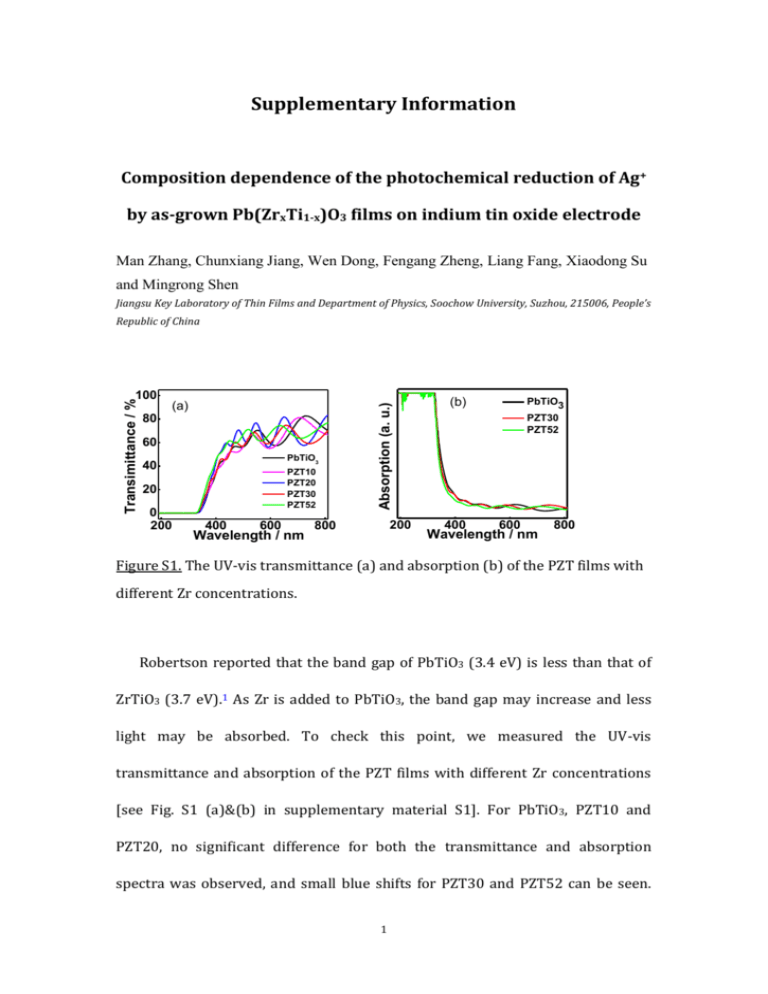
Supplementary Information Composition dependence of the photochemical reduction of Ag+ by as-grown Pb(ZrxTi1-x)O3 films on indium tin oxide electrode Man Zhang, Chunxiang Jiang, Wen Dong, Fengang Zheng, Liang Fang, Xiaodong Su and Mingrong Shen Jiangsu Key Laboratory of Thin Films and Department of Physics, Soochow University, Suzhou, 215006, People’s Transimittance / % 100 80 (a) 60 PbTiO3 40 PZT10 PZT20 PZT30 PZT52 20 0 200 400 600 Wavelength / nm Absorption (a. u.) Republic of China 200 800 (b) 400 PbTiO3 PZT30 PZT52 600 Wavelength / nm 800 Figure S1. The UV-vis transmittance (a) and absorption (b) of the PZT films with different Zr concentrations. Robertson reported that the band gap of PbTiO3 (3.4 eV) is less than that of ZrTiO3 (3.7 eV).1 As Zr is added to PbTiO3, the band gap may increase and less light may be absorbed. To check this point, we measured the UV-vis transmittance and absorption of the PZT films with different Zr concentrations [see Fig. S1 (a)&(b) in supplementary material S1]. For PbTiO3, PZT10 and PZT20, no significant difference for both the transmittance and absorption spectra was observed, and small blue shifts for PZT30 and PZT52 can be seen. 1 According to the process described in our recent work,2 we determine that the optical band gaps for PbTiO3, PZT10, PZT20, PZT30 and PZT52 are 3.47, 3.48, 3.48, 3.55 and 3.58 eV, respectively. Such results illustrate that the band gap does not change obviously when Zr concentration is within 20%. Thus the absorption can not lead to the result that the PEC photocurrent in Fig. 4(a) decreases sharply from PbTiO3 to PZT20. However, for PZT with Zr concentration larger than 20%, the change in absorption may be another reason for the change of the PEC properties. PZT are interesting since their extraordinary dielectric and piezoelectric behaviors around morphotropic phase boundary (MPB, about Zr:Ti=52:48) where the properties are enhanced.3 For Ba1-xSrxTiO3 ceramics4,5 and Gd doped BiFeO3 nanoparticles6, the reported results indicated that the high dielectric constant at the phase boundary can increase the width of the space charge region and charge carrier separation in the near surface region, and therefore enhance the photochemical processes. Our previous study showed that the dielectric constant also reaches a maximum at MPB for PZT films.7 However, in this study, we did not observe the enhancement of the PEC processes near the MPB composition, indicating that such effect may be weak enough for PZT films and/or be overweighed by other effects. 2 Figure S2. SEM images of the surface of PbTiO3 exposed in AgNO3 solution with different illumination time: (a) 5 min, (b) 10 min, (c) 15 min, and (d) 20 min. Fig. S2 presents the Ag nanoparticles photochemically grown on the as-grown PbTiO3 film surfaces at different stages. It is interesting to note from Fig. S2(a) that no any Ag nanoparticles appear on the film surface after an initial illumination of 5 min. When the illumination time is larger than 10 min, both the Ag particle size and density increase with the increase of illumination time, as shown in Fig. S2(b) to 2(d). The photocathodic behavior shown in Fig. 4(a) illustrates there is a net internal electric field in the ITO/PZT electrode with direction pointing from ITO to PZT surface, and then the Stern layer may form on PZT surface due to the surface potential that forms when the internal charge compensation process is only partially screening the internal field.8 With the aqueous AgNO3 solution the layer would consist of desolvated NO3 ions and polarized H2O molecules, which acts as a barrier to the Ag+ ions in the outer Helmholtz and diffusion region, as proposed by Dunn et al.8 In order for the Ag+ 3 ion to reach the PZT surface and nucleate there must be a disturbance in the Stern layer. Under illumination, the photoexcited electrons migrate to reduction sites on the PZT surface and accumulate, causing a localized variation in electrical potential V to a more negative one. Within the first 5 min illumination, the accumulated electrons is not large enough and thus the V is not negative enough to repel the NO3 anions and attracting the Ag+ ions. Therefore, no Ag nanoparticles can be observed. Above 5 min illumination, the accumulated electrons is larger enough to attract the Ag+ ions which subsequently accept the electron from the PZT surface, resulting in the growth of Ag nanoparticles, as illustrated in Fig. 4(b) to 4(d). However, Dunn et al.9 reported that the nucleation density does not change significantly from the initial nucleation density as the illumination time increases. This point is not consistent with our finding that both the Ag particle size and density increase with the increase of illumination time. In this study, the factor dominating the Ag growth behavior is the ITO/PZT interface barrier instead of polarized domain surface. The former may produce an internal electric field more uniformly distributed in the PZT film, making the photogenerated electrons arriving at the film surface and accumulating there more evenly and resulting in the uniform distribution of the Ag nanoparticles. Therefore, new Ag nanoparticles can nucleate on PZT surface as the illumination time increases. References: 1J. Robertson, J. Vac. Sci. Technol. B 18, 1785 (2000). 4 2D. W. Cao, C. Y. Wang, F. G. Zheng, W. Dong, L. Fang, and M. R. Shen, Nano Lett. 12, 2803 (2012). 3I. Grinberg, V. R.Cooper, and A. M.Rappe, Nature(London) 419, 909 (2002). 4A. Bhardwaj, N. V. Burbure, and G. S. Rohrer, J. Am. Ceram. Soc. 93(12), 4129 (2010). 5A. Bhardwaj, N. V. Burbure, A. Gamalski, and G. S. Rohrer, Chem. Mater. 22, 3527 (2010). 6R. Q. Guo, L. Fang, W. Dong, and F. G. Zheng, and M. R. Shen, J.Phys. Chem. C 114, 21390 (2010). 7F. G. Zheng, J. P. Chen , X. W. Li, and M. R. Shen, Mater. Lett. 60, 2733 (2006). 8P. M. Jones and S. Dunn,J. Phys. D: Appl. Phys. 42, 065408 (2009). 9S. Dunn, S. Sharp, and S. Burgess, Nanotechnology 20, 115604 (2009). 5