DyanOT_en
advertisement
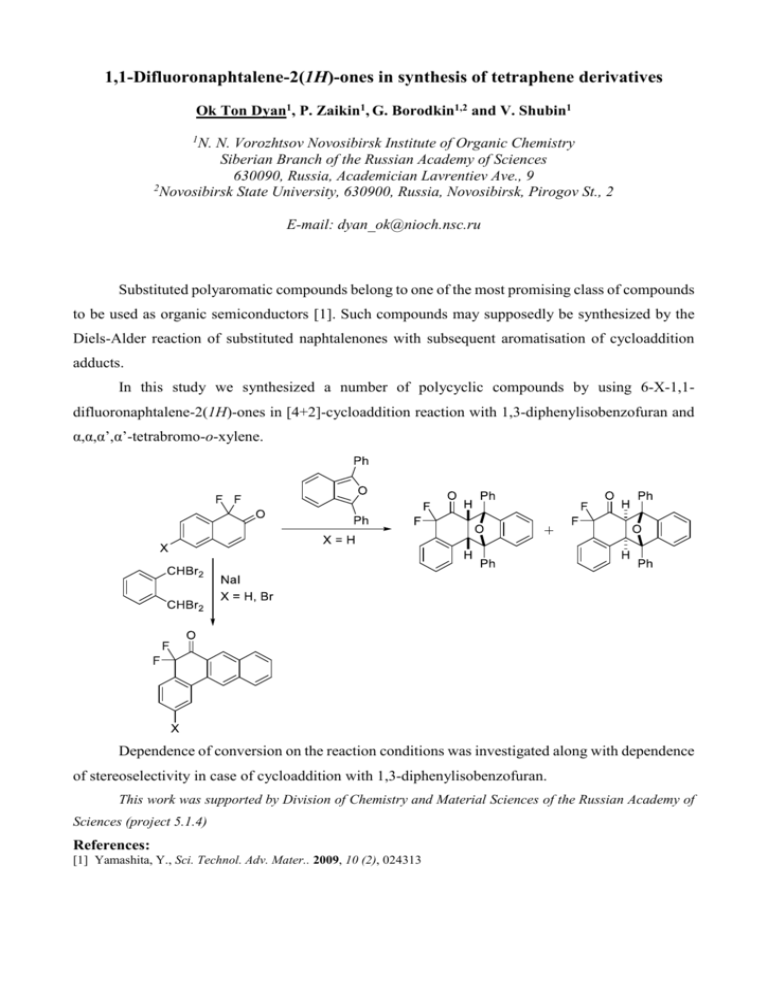
1,1-Difluoronaphtalene-2(1H)-ones in synthesis of tetraphene derivatives Ok Ton Dyan1, P. Zaikin1, G. Borodkin1,2 and V. Shubin1 1 N. N. Vorozhtsov Novosibirsk Institute of Organic Chemistry Siberian Branch of the Russian Academy of Sciences 630090, Russia, Academician Lavrentiev Ave., 9 2 Novosibirsk State University, 630900, Russia, Novosibirsk, Pirogov St., 2 E-mail: dyan_ok@nioch.nsc.ru Substituted polyaromatic compounds belong to one of the most promising class of compounds to be used as organic semiconductors [1]. Such compounds may supposedly be synthesized by the Diels-Alder reaction of substituted naphtalenones with subsequent aromatisation of cycloaddition adducts. In this study we synthesized a number of polycyclic compounds by using 6-X-1,1difluoronaphtalene-2(1H)-ones in [4+2]-cycloaddition reaction with 1,3-diphenylisobenzofuran and α,α,α’,α’-tetrabromo-o-xylene. Dependence of conversion on the reaction conditions was investigated along with dependence of stereoselectivity in case of cycloaddition with 1,3-diphenylisobenzofuran. This work was supported by Division of Chemistry and Material Sciences of the Russian Academy of Sciences (project 5.1.4) References: [1] Yamashita, Y., Sci. Technol. Adv. Mater.. 2009, 10 (2), 024313











