States of Matter: Physics Essay on Plasmas, Gases, Liquids, Solids
advertisement

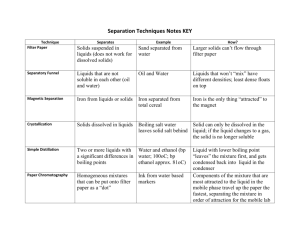
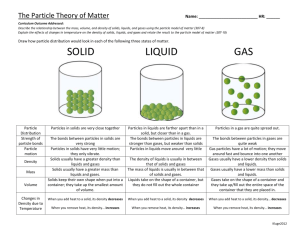
Shea Anderson, Jack Alexander, Martin Shea, Neal Sanders, Tori Schaus Physics Essay Period 5 STATES OF MATTER Matter is made up of elements and compounds that occupy space, and one can change the physical state of said matter by applying physical forces like changing temperature and pressure. There are five states of matter: plasmas, gases, liquids, solids, and Bose-Einstein condensates. We are most familiar with gases, liquids, solids, and plasmas as we come in contact with them every day. BoseEinstein condensates aren’t very common here on Earth (or in the universe), so we tend to think less of them. Plasmas are found in the stars of the universe, while Bose-Einstein condensates only occur in the darkest depths of space. If you were to think of the states of matter as a spectrum, plasmas and BoseEinstein Condensates are found on the very ends. Gases and Solids move closer to the middle, and liquids are perhaps the most important right in the center. If it weren’t for liquids, life in the universe would not be possible. Let us start from the middle of our spectrum: liquids. Liquids, like a gas, have an indefinite shape; but like a solid, have definite volume. Liquids are forms of matter that are made up of more tightly packed particles, but are not placed in regular patterns, giving them the ability to move and conform to the shape of the container they are held in. The density of liquids is very close to that of a solid and both are described as condensed matter. From the liquid state we can apply temperature to change its physical state. If we apply heat, the liquid molecules will grow farther apart, to the point where the cohesive forces of the molecules break, changing into a gaseous state. If we apply cold temperatures, the distances between molecules grow smaller, to where it will freeze, making the bonds more rigid. Moving outward from the middle of our spectrum we find gases and solids. When heat is applied to a liquid, it undergoes evaporation, moving from a liquid state of matter to a gaseous state. In this state, the matter expands to occupy the available volume. Gases are forms of matter with fast moving particles further spread out from each other. Gasses expand and compress to fit an area. Just as liquid can turn into a gaseous state, gas can turn into a liquid state by condensation: we often see this process in rainfall. When a gas is exposed to below freezing temperatures, it can turn into a solid state without becoming a liquid first. This process is called deposition: and most commonly seen in the process of snowfall. The process in which a solid become a gas without becoming a liquid is called sublimation. When a liquid is cooled down, it freezes into a solid state of matter. Solids have a definite shape and self contained form. They are made up of regularly patterned, tightly packed particles that can only vibrate in their fixed positions, and cannot move freely and flow like a liquid or gas. Solids can only change their shape by an applied force like breaking or cutting. Plasma is electrically charged particles at high energy, being the hottest state of matter that is very similar to a gas. The difference between plasma and gas is a certain portion of the particles are ionized. This happens because heating a gas dissociates its molecular bonds, freeing the atoms, losing its electrons and becoming positively charged. We see plasma throughout the universe in stars, and very often here on Earth with the glow coming from fluorescent lights. Due to plasma having an electric current, plasmas are more influenced by electromagnetic forces rather than gravitational forces. Throughout the universe, the most dominant form of matter happens to be plasma, and plasma cooling down gives all the atoms and molecules that form gases, liquids, and solids. So, one could reasonably argue that we are all made of stars. Further cooling down from a solid, we find the infamous Bose-Einstein Condensate. Closing in on the temperature of absolute zero, a mysterious thing happens to matter: it turns into a super liquid. A liquid with no viscosity (the ability to flow without dissipating energy), and its particles are so still, it is able to slip through the atoms surrounding it. The finding of this state of matter allows us to see quantum events on a macroscopic level, furthering our understanding of the universe. Yet these condensates can only be observed in short periods of time; therefore, research is currently limited, making it all the more difficult to explain quantum events. The way we, the human race, understand the states of matter around us, will further increase the understanding of our surrounding universe. Knowing how matter moves and how it works, gives us insight to what we are able to learn about far off planets, and the origins of the universe. Through BoseEinstein Condensate and plasma research, we peer into bits of quantum chaos in hopes of finding how everything has come into existence, and where it has brought us into the present.