Effect of nitrile-functionalization of imidazolium
advertisement

Effect of nitrile-functionalization of imidazolium-based ionic liquids on their
transport properties pure and mixed with lithium salts.
Hassan Sroura,d, Mounir Traïkiab,c, Bernard Feneta, Hélène Rouaultd, Margarida F.
Costa Gomesb, Catherine C. Santinia, Pascale Hussonb,c*
Supporting Information Available
Synthesis of [C1C4Im]Cl and [C1C4Im][NTf2]
[C1C4Im]Cl 1-chlorobutane (106 mL, 1.01 mol) was added to freshly distilled 1methylimidazole (50 mL, 0.63 mol). The mixture was stirred for 48 h at 65°C. The hot
solution was then transferred drop wise via a cannula into toluene (200mL) at 0°C
under vigorous mechanical stirring. The white precipitate formed was then filtered
and washed repeatedly with toluene (3×200 mL) and dried overnight in vacuo giving
a white powder (95.6 g, 87 %).1H-NMR (CD2Cl2): δ (ppm) : 11.05 (s, 1H, C2H) ; 7.33 (d,
1H, C4H) ; 7.28 (d, 1H, C5H) ; 4.31 (t, 2H, NCH2) ; 4.07 (s, 3H, NCH3) ; 1.90 (qt, 2H,
CH2CH2CH2) ; 1.41 (st, 2H, CH2CH2CH3) ; 0.96 (t, 3H, CH2CH3) ; 13C{1H}-NMR (CD2Cl2) :
δ (ppm) : 138.3 (C2H) ; 122.3 (C4H) ; 119.8 (C5H) ; 50.1 (NCH2) ; 36.8 (NCH3) ; 32.5
(CH2CH2CH2) ; 19.8 (CH2CH2CH3) ; 13.6 (CH2CH3).
[C1C4Im][NTf2] A solution of Li[NTf2] (50 g, 0.17 mol) in water (50 mL) was added to a
solution of [C1C4Im]Cl (30.4 g, 0.17 mol) in water (100 mL). The solution was stirred
for 2 h at room temperature, then dichloromethane (50 mL) was added and the
mixture transferred to a separating funnel. The lower phase was collected and
washed repeatedly with water (8 × 100 mL) until no chloride traces remained in the
washings (tested with silver nitrate solution). The ionic liquid in dichloromethane
was purified through a short alumina column and the solvent removed in vacuo
giving a colourless viscous liquid. 1H-NMR (CD2Cl2): δ (ppm) : 8.73 (s, 1H, C2H) ; 7.28
(d, 1H, C4H) ; 7.24 (d, 1H, C5H) ; 4.15 (t, 2H, NCH2) ; 3.90 (s, 3H, NCH3) ; 1.83 (qt, 2H,
CH2CH2CH2) ; 1.32 (st, 2H, CH2CH2CH3) ; 0.94 (t, 3H, CH2CH3) ; 13C{1H}-NMR (CD2Cl2) :
δ (ppm) : 134.3 (C2H) ; 124.3 (C4H) ; 122.4 (C5H) ; 118.2 (CF3) ; 50.3 (NCH2) ; 37.1
(NCH3) ; 33.2 (CH2CH2CH2) ; 19.8 (CH2CH2CH3) ; 13.5 (CH2CH3).
S1
Synthesis of [C1C3CNIm]Cl and [C1C3CNIm][NTf2]
[C1C3CNIm]Cl. A mixture of 1-methylimidazole (20g, 244 mmol) and Cl(CH2)3CN
(29,5g, 293 mmol) was stirred at 353 K for 24h. Diethyl ether (40 mL) was
added at room temperature to the oily liquid, the mixture was stirred for 2
hours. The resulting white-yellow solid was washed with diethyl ether (3x30
mL). The solvent was then removed via rotary evaporation. The solid product
was dried under vacuum. The product yield is quantitative. 1H NMR (300 MHz,
CD2Cl2): 9,46 (s, 1H); 7.86 (t, 1H) ; 7.73 (t, 1H) ; 4.24 (t, J=9Hz, 2H) ; 3.80 (s,
3H) ; 2.26 (t, J=9Hz, 2H) ; 2.08 (q, J=6Hz, 2H).13C NMR (75 MHz, CD2Cl2): 13.63;
26.11; 36.11; 47.73; 120.47; 122.75; 124.26; 137.39. HRMS-ESI (m/z):
calculated as [A] +, [C8H12N3] + = 150.1024; found, 150.1026.
[C1C3CNIm][NTf2] [Li][NTf2] (34g, 119 mmol) was added at 298 K to a solution
of [C1C4Im]Cl (20g, 108 mmol) in distilled water 70 mL, and an oily liquid could
be immediately separated. After stirring the reaction mixture for 2 hours, the
product was extracted into ethyl acetate (50 mL) and the organic phase was
washed with distilled water (3x50 mL). The solvent was removed by rotary
evaporation. The final pale-yellow product was dried under vacuum. The
product yield is 65%. 1H NMR (300 MHz, DMSO-d6): 9.01 (s, 1H) ; 7,61 (t, 1H) ;
7,53 (t, 1H) ; 4.19 (t, J= 9Hz, 2H) ; 3.78 (s, 3H) ; 2.48 (t, J= 6Hz, 2H) ; 2,11 (q, J=
6Hz, 2H). 13C{1H}-NMR (d6-DMSO): (CH3, 36.16); (CH2, 13.77, 25.49, 48.34);
(CH, 119.23; 121.89, 137.12); (CF3, q, 113.41; 117.79; 119.25; 125.99); (CN,
123.79). HRMS-ESI (m/z): calculated as [C18H24N7F6O4S2]+ = 580.1209; found,
580.1230. IR (cm-1): 2251 (υ CN)
S2
Table S1 Parameters a and b of Eq. 2, used to fit the experimental densities from
Table 1 as a function of temperature along with the Standard Error of the Estimation
(SEE) (standard deviation between the experimental and calculated densities).
[C1C4Im][NTf2]
[C1C3CNIm][NTf2]
Pure
cLi[NTf2] = 1 mol·L−1
Pure
cLi[NTf2] = 1 mol·L−1
a / kg·m–3·K–1
– 0.935
– 0.982
– 0.901
– 0.919
b /kg·m–3
1712.47
1791.71
1779.38
1844.07
SEE
0.5
0.4
0.3
0.2
Table S2 Experimental viscosities (mPa·s) of pure ILs and their mixtures with
[Li][NTf2] as a function of temperature at atmospheric pressure
[C1C4Im][NTf2]
Pure
[C1C3CNIm][NTf2]
cLi[NTf2] =1 mol·L−1
Pure
cLi[NTf2]= 1 mol·L−1
/mPa·s
T /K
298.15
51.1
163
208
825
313.15
28.7
76.2
92.9
297
333.15
15.9
33.0
40.9
95.5
353.15
9.75
18.1
21.8
48.5
373.15
6.46
11.4
13.4
26.2
Standard uncertainties u are u(T) = ± 0.01 K, u() = ± 1.5 %
S3
Table S3 Parameters A, k and T0 of eq 3, used to fit the experimental viscosities from
Table S2 as a function of temperature along with the Standard Error of the
Estimation (SEE).
[C1C4Im][NTf2]
[C1C3CNIm][NTf2]
Pure
cLi[NTf2] = 1 mol·L−1
pure
cLi[NTf2] = 1 mol·L−1
5.47
3.53
8.24
2.83
k/K
903
1063
848
1203
T0 / K
155
163
182
175
SEE
0.1
0.5
0.05
3
A
/10–3
mPa·s·K1/2
S4
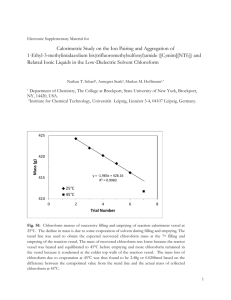
Table S4 Experimental electrical conductivities () of the pure ILs and their mixtures
with Li[NTf2] as a function of temperature
[C1C4Im][NTf2]
cLi[NTf2] = 1 mol·L-1
Pure
T/K
/ mS·cm–1
297.95
4.01
312.77
104
/
104
T/K
/ mS·cm–1
1.17
297.98
1.55
0.403
6.95
2.05
312.89
3.11
0.817
332.56
12.42
3.72
332.79
6.41
1.70
352.61
19.61
5.95
352.74
11.18
3.01
372.43
28.32
8.71
372.6
17.37
4.75
S·m2·mol–1
/
S·m2·mol–1
[C1C3CNIm][NTf2]
cLiNTf2 = 1 mol·L-1
Pure
T/K
/ mS·cm–1
298.4
1.25
312.85
104
/
104
T/K
/ mS·cm–1
0.356
297.86
00.31
0.079
2.63
0.755
312.59
0.83
0.212
332.84
5.84
1.70
332.3
2.25
0.582
352.65
10.77
3.17
352.43
4.78
1.25
372.49
17.51
5.22
372.2
8.58
2.28
S·m2·mol–1
/
S·m2·mol–1
Standard uncertainties u are u(T) = ± 0.01 K, u() = ± 0.6 %, u() = ± 2 %
S5
Table S5 Parameters A’, k’ and T0’ of Eq. 3, used to fit the experimental electrical
conductivities from Table S4 as a function of temperature along with the Standard
Error of the Estimation
[C1C4Im][NTf2]
[C1C3CNIm][NTf2]
pure
cLiNTf2 = 1 mol·L−1
Pure
cLiNTf2 = 1 mol·L−1
32.00
30.08
48.44
38.47
k’ / K
–614.6
–660.3
–759.6
–793.2
T 0’ / K
173.2
184.4
181.2
194.3
SEE
0.005
0.0003
0.007
0.0007
A’
/S·m-1·K1/2
S6