Chemistry Summer Assignment
advertisement

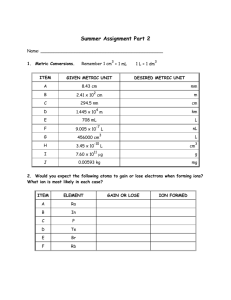
Chemistry II Summer Homework Assignment Name: _______________________________________________ 1. Metric Conversions. Remember 1 cm3 = 1 mL GIVEN METRIC UNIT 8.43 cm 1 L = 1 dm3 DESIRED METRIC UNIT mm 2.41 x 102 cm m 294.5 nm cm 1.445 x 104 m km 708 mL L 9.005 x 10-7 L nL 456000 cm3 L 3.45 x 10-10 L cm3 7.60 x 1011 µg g 0.00593 kg mg 2. Would you expect the following atoms to gain or lose electrons when forming ions? What ion (symbol and charge) is most likely in each case? ELEMENT K Ba P O Br Rb GAIN OR LOSE ION FORMED 3. Chemical Nomenclature: You should be able to complete this using a periodic table and MINIMAL help from other sources. ITEM A B C D E F G H I J K L M N O P Q R S T U V W X Y Z AA BB CC DD EE FF GG HH II JJ CHEMICAL FORMULA NaBr CaS AlI3 CHEMICAL NAME Strontium fluoride Potassium nitride Magnesium phosphide Hg2O FeBr3 CoS TiCl4 Tin (II) nitride Cobalt (III) iodide Mercury (II) oxide Chromium (VI) sulfide BaSO3 NaNO2 KMnO4 K2Cr2O7 Chromium (III) hydroxide Magnesium cyanide Lead (IV) carbonate Ammonium acetate N2O4 ICl3 SO2 P2S5 Diboron trioxide Arsenic pentafluoride Dinitrogen monoxide Sulfur hexachloride HC2H3O2 H3PO3 HCl Hydrofluoric acid Sulfurous acid Phosphoric acid 4. Chemical Reactions – Write and balance the following reactions. a. Glucose (C6H12O6) reacts with oxygen gas to produce gaseous carbon dioxide and water vapor. b. Solid iron (III) sulfide reacts with gaseous hydrogen chloride to form solid iron (III) chloride and hydrogen sulfide gas. c. Carbon disulfide liquid reacts with ammonia gas to produce hydrogen sulfide gas and solid ammonium thiocyanate (NH4SCN). d. Aqueous solutions of lead (II) nitrate and sodium phosphate are mixed resulting in the precipitate formation of lead (II) phosphate and aqueous sodium nitrate as the other product. e. Solid zinc reacts with aqueous hydrochloric acid to form aqueous zinc chloride and hydrogen gas. f. Aqueous calcium hydroxide is neutralized by aqueous phosphoric acid to produce solid calcium phosphate and water. 5. Chemical Calculations – You must show the work (on a separate sheet of paper) that leads to your answer to receive credit. Write your answers in the blanks to the left of the question. Remember that:1 mole element = 6.022 x 1023 atoms 1 mole compound = 6.022 x 1023 molecules or formula units ❖ molecules refer to covalent compounds while formula units refer to ionic compounds (mathematically they mean the same thing) A. Bauxite, the principle ore used in the production of aluminum, has the molecular formula of Al2O3·2H2O (aluminum oxide dihydrate). ____________ (i) What is the molar mass of bauxite? ____________ (ii) What is the mass of aluminum in 0.580 mol bauxite? ____________ (iii) How many atoms of aluminum are in 0.580 mol bauxite? ___________ (iv) What is the mass of 2.10 x 1024 formula units of bauxite? B. Chloral hydrate (C2H3Cl3O2) is a drug formerly used as a sedative and hypnotic. ____________ (i) Calculate the molar mass of chloral hydrate. ___________ (ii) How many moles of chloral hydrate molecules are in 500.0 g of chloral hydrate? ___________ (iii) What is the mass in grams of 2.00 x 10-2 mol chloral hydrate? ___________ (iv) What number of chlorine atoms are in 5.00 g chloral hydrate? ___________ (v) What mass of chloral hydrate would contain 1.00 g Cl? ___________ (vi) What is the mass of exactly 500 molecules of chloral hydrate? 6. Stoichiometry –Show your work on a separate sheet of paper but write the answer in the blank to the left of the question. Remember the equation must be balanced. A. The reaction between potassium chlorate and red phosphorus (P4) takes place when you strike a match on a matchbox. The products are tetraphosphorus decaoxide and potassium chloride. Answer the following questions using this reaction. (i) Write the balanced chemical equation for this reaction. _________(ii) What mass of tetraphosphorus decaoxide is formed when 52.9 g of potassium chlorate reacts with an excess of red phosphorus? ________ (iii) If the reaction produces 32.5 grams of tetraphosphorus decaoxide, what is the percent yield? B. Phosphorus (P4) can be prepared from calcium phosphate by the following reaction: Ca3(PO4)2 + SiO2 + C CaSiO3 + P4 + CO (i) Balance the equation. [Hint: start with phosphorus] _________ (ii) How many grams of carbon is need to react with 73.5 mg of silicon dioxide? _________ (iii) Phosphorite is a mineral that contains Ca3(PO4)2 plus other non-phosphorus containing compounds. What is the maximum amount of P4 that can be produced from 1.00 kg of phosphorite if the phosphorite sample is 75% calcium phosphate by mass? Assume an excess of the other reactants. C. 500 g sulfuric acid reacts with 400 g barium hydroxide producing a barium sulfate solid precipitate and water. (i)Write and balance the equation for this reaction. _________(ii) Determine the limiting reactant. _________(iii) Calculate the mass of water produced. _________(iv) Calculate the mass of the excess reactant remaining after the reaction goes to completion. Solubility Rules 1. All compounds containing alkali metal cations and the ammonium ion are soluble. 2. All compounds containing nitrate, perchlorate, chlorate, and acetate ions are soluble. 3. All chlorides, bromides, and iodides are soluble except those containing 1+ Ag , Pb2+, or Hg22+. 4. All sulfates are soluble except those containing Hg22+, Pb2+, Sr2+, Ca2+, or Ba2+. 5. All hydroxides are insoluble except compounds of the alkali metals, 2+ Ca , Sr2+, and Ba2+. 6. All compounds containing PO43-, S2-, CO32-, and SO32- ions are insoluble except those that also contain alkali metals or NH41+. SOLUBLE INSOLUBLE Alkali metal cations (these are group I metal ions) Hydroxides (except: alkali metals, ammonium, Ca+2, Sr+2, Ba+2) Ammonium ion Phosphates, Sulfides, Carbonates, Sulfites, Chromates (except: alkali metals and ammonium) Nitrates, Perchlorates, Chlorates, Acetates Chlorides, Bromides, Iodides (except: Ag+1, Pb+2, Hg22+) Sulfates (except: Pb+2, Hg22+, Sr+2, Ca+2, Ba+2) Both of these have the same information just arranged in a different way. You need to begin learning these rules. We will begin using these the first week of school and quizzes related to this information will begin by the second week of school. You should also know ALL of the polyatomic ions on the next page. Pay attention to those ions ending in –ite versus – ate. You learned these in Chem I with me. (CLO)-1 HYPOCHLORITE (BRO)-1 HYPOBROMITE (IO)-1 HYPOIODITE (ClO2)-1 chlorite (BrO2)-1 bromite (IO2)-1 iodite (ClO3)-1 chlorate (BrO3)-1 bromate (IO3)-1 iodate (ClO4)-1 perchlorate (BrO4)-1 POLYATOMIC ION NAME Ammonium Acetate Cyanide Hydrogen carbonate Hydrogen sulfate Hydroxide Nitrate Nitrite Hypochlorite Chlorite Chlorate Perchlorate Permanganate Iodate Thiocyanate Oxalate Carbonate Chromate Dichromate Sulfate Sulfite Thiosulfate Arsenate Phosphate Phosphite perbromate (IO4)-1 periodate SYMBOL and OXIDATION NO. ( NH4 ) + 1 (CH3COO) –1 or ( C2H3O2) –1 ( CN ) - 1 ( HCO3 ) -1 ( HSO4 ) - 1 ( OH ) -1 ( NO3 ) -1 ( NO2 ) -1 ( ClO ) -1 ( ClO2 ) -1 ( ClO3 ) -1 ( ClO4 ) -1 ( MnO4 ) -1 ( IO3 ) -1 ( SCN ) - 1 ( C2O4 ) - 2 ( CO3 ) -2 ( CrO4 ) -2 ( Cr2O7 ) -2 ( SO4 ) -2 ( SO3 ) -2 ( S2O3 ) -2 ( AsO4 ) -3 ( PO4 ) -3 ( PO3 ) –3 These problems are all based upon concepts and skills that you learned (or should have) in Chem I. We will only spend the first 2 weeks reviewing this material. If you couldn’t finish this assignment with a periodic table and a calculator (with 1 or 2 googles allowed-I realize it’s summer), you will find Chem 2 an uphill climb. I will not be starting from scratch and reteaching you Chem I as if you’d never seen it before. We will review and refresh your memory. In addition, the problems we work involving these Chem I topics are written on a much higher level than these (which are similar to the ones you worked in my Chem I class). By the third or fourth week of school, we will move into new topics which will build upon these concepts and skills as well as add new ones. The course moves at a college-level pace and you will be expected to keep up with homework, write lab reports and perform calculations independently, seek help (I will not chase you). I am happy to see you during my planning period, during lunch, or before school if you have questions. GOOD NEWS: No lengthy projects or research papers, just practice problems and be sure you know what you are doing! If you pay good attention in class, DON’T MISS CLASS, and keep well-organized notes, your homework should not take that long. If I recommended that you take this course, it is because I believe you are capable of being successful in it. As a college-bound student, you should appreciate the challenge offered in Chem 2. We will work hard, but we will also have fun. Plus, there is the added ego boost of knowing you took one of the hardest classes in high school, ACED IT and got Purdue credits, too! See you in August!! Oxygen and Potassium went on a date… It went OK. :D