summary - Inside Mines
advertisement

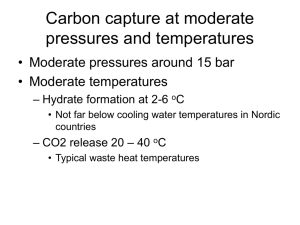
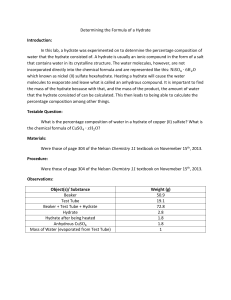
Marisa B. Rydzy Statistical Thermodynamics “Model for Gas Hydrate Equilibria using a Variable Reference Chemical Potential” by Lee & Holder (2002) Gas Hydrates Clathrate hydrates are nonstoichiometric ice-like crystalline solids comprised of a threedimensional network of hydrogen-bonded water molecules, which confines guest molecules in well-defined cavities. Gas Hydrates are stable only at conditions of low temperatures and elevated pressures. Being able to predict gas hydrate stability pressure and temperatures is of vital importance to the oil and gas industry as hydrate plug formation in pipelines can cause severe damage. The paper published by Lee & Holder (2002) is mainly concerned with the prediction of gas hydrate equilibrium pressures applying a statistical thermodynamics model. Correlation of experimental and theoretical data is then successfully used to demonstrate the validity of the model. The Model The model presented by Lee & Holder is based on the statistical thermodynamic model for describing gas hydrate phase equilibrium, which was developed, generalized, and further modified by Van der Waals & Platteeuw (1959), Parrish & Prausnitz (1972) and Holder et al. (1981), respectively. The model describes the hydrate phase, when liquid water is present and assumes that a) classical statistical thermodynamics is valid, b) one cavity can only accommodate one molecule, and c) the mutual interaction between enclathrated molecules is negligible. Unlike Van der Waals and Platteeuw who assumed a rigid cage in which the guest rotates freely; Lee & Holder assumed that the size of guest molecules does indeed impact the host-host interactions by distorting the lattice, thus changing the theoretical chemical potential of the theoretical (empty) lattice. To account for this, the authors introduce a guest depended reference chemical potential difference, which is derived from experimental data and can be used to predict equilibrium pressure and temperatures for simple gas hydrates. An empirical correlation relating cage radius and the reference chemical potential difference as well as smooth cell potentials are integrated into the equations determining the Langmuir constants to account for the lattice distortion as well as as for the contribution of more distant water molecules and the distortion of the lattice, respectively. Application At equilibrium the chemical potential differences (the chemical potential of the theoretical empty lattice serves as a reference state) of the pure liquid water and the hydrate are equal. Following the recipe given below, the chemical potential differences of the pure water and the hydrate phase can be calculated. Thereby an initial value for the reference chemical potential difference is assumed for 273.15 K and the respective (experimentally derived) dissociation pressure. If the chemical potential at the end of the calculation are not equal, a new reference chemical potential difference, using an appropriate convergence method, i.e. the calculations are repeated until the two chemical potential differences become identical. I. Calculating the guest-dependent reference chemical potential of the hydrate phase a. A first approximation for the guest-dependent reference chemical potential is assumed based on experimentally obtained dissociation pressure data at 273.15 K b. The guest-dependent cavity radii are calculated for each cell using the guestdependent reference chemical potential. c. The Langmuir Constants for the respective cages are calculated from the radii for the small and large cages. Here, the smooth cell potentials are incorporated. d. The Langmuir Constants are then used to determine the fractional cage occupancies of i-type cavities with j-type guests using fugacity coefficients fj which are using Peng-Robinson Equation. e. Knowing the fractional occupancy of the hydrate, the chemical potential difference of the hydrate phase can be calculated using the VdWP Model. 2 Hw (T, P) RT i ln 1 j (1) i 1 j II. Calculating the guest-dependent reference chemical potential of the liquid phase a. To obtain the guest-dependent reference enthalpy difference Equation (1) and (3) are set equal, rearranged and the integrals solved with appropriate limits. The result is a linear equation of the form Y=αX, where the term α includes the reference enthalpy difference between theoretical empty hydrate cage and the pure water phase at 273.15 K. The slope of the graph X vs. Y thus yields the reference enthalpy difference for the liquid water phase. The method to calculate the heat capacity difference and b is given elsewhere. 1 h 0w C 0pw T0 bT02 2 (2) b. The reference enthalpy difference for the liquid water phase is then used to calculate the temperature-dependent enthalpy, which is thereafter applied to determine the guest-dependent reference chemical potential of the liquid phase given by Equation (8) P 0w T h w Vw RT dT dP ln w w 2 RT P0 RT0 T0 RT L w (3) [Most of the equations presented by Lee and Holder have been omitted from this review to not exceed the two page limit.]