Biochemistry Ch 20 355-375
advertisement

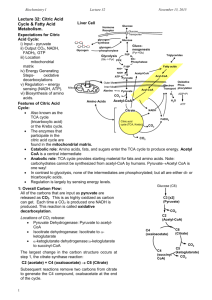
Biochemistry Ch 20 355-375 Tricarboxylic Acid Cycle – called Krebs cycle and the citric acid cycle -Substrate for TCA cycle is acetyl CoA which combines with oxaloacetate to form citrate (6C) -electrons are transferred in the next two reactions to form NADH and CO2, followed by a GTP produced -Succinate Fumarate causes FADH2 to be produced followed by NADH from malate oxaloacetate -TCA requires vitamins and minerals: NAD, riboflavin, FAD and FMN, CoA, thiamine, Mg, Ca, Fe, PO4 NET REACTION: Ac-CoA + 3 NAD + FAD 2CO2 + CoASH + 3NADH + 3H + FADH2 + GTP Reactions of the TCA Cycle – the 2 carbons of acetyl-CoA are oxidized to 2 CO2 -energy is stored through NAD and FAD, where 8 electrons donated form 3 NADH and 1 FADH2 and ATP can be made through OxPhos -The O2 in the 2 CO2’s comes from acetyl group + carbonyl group of acetyl CoA -2.5 ATP generated per NADH and 1.5 ATP for each FADH2 -net energy yield from TCA and OxPhos is 10 phosphate bonds for each acetyl group oxidized A. Formation and Oxidation of Isocitrate – TCA begins with activated acetyl group and oxaloacetate to form citrate, catalyzed by citrate synthase -in the next step, OH is isomerized to a different carbon forming a keto group called isocitrate by an enzyme aconitase -subsequent cleavage of isocitrate to α-ketoglutarate by isocitrate dehydrogenase releases CO2 Summary: Acetyl CoA + Oxaloacetate (citrate synthase)Citrate (Aconitase) isocitrate (isositrate dehydrogenase) α-ketoglutarate + CO2 B. α-ketoglutarate to Succinyl CoA – catalyzed by α-ketoglutarate dehydrogenase which contains coenzymes thiamine phosphate, lipoic acid, and FAD -one CO2 is released, and the adjacent keto group is oxidized to an acid, combining with CoA to form succinyl coA Summary: α-ketoglutarate (α-ketoglutarate dehydrogenase) Succinyl CoA C. Generation of Guanosine Triphosphate - energy from succinyl CoA thioester bond is used to generate GTP from GDP by reaction with succinate thiokinase or succinyl-CoA synthetase (for reverse reaction). This is substrate-level phosphorylation (no O2 used) Summary: Succinyl CoA + GDP (succinate thiokinase)Succinate + GTP D. Oxidation of Succinate to Oxaloacetate – these steps transfer two pairs of electrons to FAD and NAD+ and add H2O to regenerate oxaloacetate -Succinate Fumarate through succinate dehydrogenase transfers electrons to FAD FADH2 -Fumarate Malate through addition of H2O by fumarase -Malate Oxaloacetate by malate dehydrogenase forms an NADH from NAD and restarts TCA Summary: Succinate + FAD (Succinate dehydrogenase) FADH2 + Fumarate (Fumarase) Malate + NAD (Malate dehydrogenase) Oxaloacetate + NADH Coenzymes of TCA Cycle – -isocitrate dehydrogenase and malate dehydrogenase::: NAD+ as a coenzyme, -succinate dehydrogenase:: FAD+ as coenzyme -citrate synthase:: Acetyl CoA as coenzyme -α-ketoglutarate:: TPP, Lipoate, FAD as bound coenzymes… NAD and CoASH as substrates 1. Flavin Adenine Dinucleotide and Nicotinamide Adenine Dinucleotide – FAD and NAD are electron-accepting coenzymes -FAD is able to accept single electrons (H*), forming free radical that must be bound tightly to enzyme while it accepts and transfers electrons to another group -NAD accepts a pair of electrons (H-), and is much more like substrate/product than coenzyme -NADH plays regulatory role in balancing energy metabolism that FADH2 cannot because FADH2 remains attached to enzyme -NADH/NAD ratio can activate and inhibit TCA cycle (NADH inhibits) 2. Role of Coenzyme A in TCA Cycle – CoASH participates in reactions by forming thioester bond between sulfer of CoASH and an acyl group on enzyme -CoASH comes from a vitamin precursor (pantothenate) -energy from the bond between succinyl-CoA and acetyl-CoA when cleaved (such as by succinate thiokinase for succinyl coA transfers Pi to GDP GTP) 3. The α-keto Acid Dehydrogenase Complexes – the α-keto acid is decarboxylated and oxidized to the level of a carboxylic acid and combined with CoASH to form acyl-CoA thioester, such as succinyl coA through a series of reactions and different enzymes -all α-keto acid dehydrogenase complexes are subunits of three different enzymes: 1. E1 - α-keto acid decarboxylase containing TPP, cleaves off carboxyl group 2. E2 – tranacylase-containing lipoate, transfers acyl portion of α-keto acid from thiamine to CoASH 3. E3 – dihydrolipoyl dehydrogenase contains FAD and transfers electrons from reduced lipoate to NAD+ -Thiamine Pyrophosphate in the α-ketoglutarate dehydrogenase complex – TPP synthesized from thiamine by addition of pyrophosphate -TPP functions to cleave C-C bond next to keto group (α-ketoglutarate, pyruvate) -TPP is also coenzyme for transketolase in the pentose phosphate pathway -in thiamine deficiency, α-ketoglutarate, pyruvate, and other α-keto acids accumulate in the blood -Lipoate – coenzyme found only in α-keto acid dehydrogenase complexes and is synthesized from carbs and amino acids; does not need vitamin precursor -at its functional end, lipoate contains disulfide group that accepts electrons when it binds acyl fragment of α-ketoglutarate, and can transfer electrons to FAD -Flavin Adenine Dinucleotide and Dihydrolipoyl Dehydrogenase – FAD on dihydrolipoyl dehydrogenase accepts electrons and transfers them to NAD -FAD accepts and transfers electrons without leaving binding site on enzyme’ -Arsenic poisoning is caused by presence of arsenious compounds that are metabolic inhibitors. Arsenate (AsO4) and Arsenite (AsO3, 10x more toxic than arsenate) bind –SH groups on dihydrolipoate and in cysteins on a-ketoacid dehydrogenase complexes and in succinic dehydrogenase. Arsenic also inhibits glyceraldehyde 3-P dehydrogenase from glycolysis Energetics of TCA Cycle – TCA operates with an overall negative ΔG; energetically favorable -some reactions, like malate dehydrogenase has a positive value of ΔG 1. Overall Efficiency of TCA Cycle – total amount of energy from acetyl group is 228 kcal/mol -products of TCA cycle (NADH, FADH2, GTP) contain 207 kcal/mol -thus, TCA cycle conserves 90% of energy available from oxidation of acetyl CoA 2. Thermodynamically and Kinetically Reversible and Irreversible Reactions – -three reactions in TCA have large negative ΔG values that strongly favor forward reactions -citrate synthase, isocitrate dehydrogenase, a-ketoglutarate dehydrogenase -these are strongly irreversible because products do not rise enough in concentration to overcome large negative ΔG and enzymes in reverse reaction act slowly -reactions by aconitase and malate dehydrogenase have a positive ΔG for forward reaction and are reversible -aconitase is rapid in both directions, so equilibrium is maintained, and citrate is 20x as concentrated as isocitrate, can be used elsewhere: fatty acid/cholesterol synthesis -malate dehydrogenase favors malate over oxaloacetate, resulting in low oxaloacetate that is influenced by NADH/NAD ratio Regulation of TCA Cycle – cells maintain ATP homeostasis by regulating rate of TCA cycle. -two major messengers feed information on rate of ATP utilization back to TCA: 1. phosphorylation state of ATP (ATP and ADP levels) 2. reduction state of NAD+ (ratio of NADH/NAD+) -cell responds so quickly to increased ATP demand that ATP concentration does not change 1. Regulation of Citrate Synthase – first step must be regulated so that precursors flow into alternative pathways if product is not needed -citrate synthase has no allosteric regulators, and is controlled by concentration of oxaloacetate (reactant) and concentration of citrate (product) which are competitive inhibitors -malate-oxaloacetate equilibrium favors malate, so oxaloacetate concentration is low -when NADH/NAD ratio decreases, ratio of oxaloacetate:malate increases -when isocitrate dehydrogenase is activated, concentration of citrate decreases, relieving product inhibition of citrate synthase -thus, both increased oxaloacetate and decreased citrate regulate response of citrate synthase to conditions established by electron transport chain 2. Allosteric Regulation of Isocitrate Dehydrogenase – metabolic pathways are regulated such that it occurs at the enzyme that catalyzes the rate-limiting step in a pathway -isocitrate dehydrogenase is a rate-limiting step of TCA cycle, and is allosterically activated by ADP, and inhibited by NADH -without ADP, enzyme exhibits positive cooperativity and all subunits are active and isocitrate binds more readily, also activated by Ca2+ 3. Regulation of a-Ketoglutarate Dehydrogenase – this complex is not an allosteric enzyme, but is inhibited by NADH and succinyl-CoA and maybe GTP -activated by Ca2+ 4. Regulation of TCA Intermediates – ensures NADH is generated fast enough to maintain ATP homeostasis and regulates concentration of TCA cycle intermediates Precursors to Acetyl CoA – compounds enter TCA as acetyl-CoA or as an intermediate that can be converted to malate/oxaloacetate -compounds entering as acetyl-CoA get oxidized to CO2, compounds entering as intermediates replenish lost intermediates to other pathways such as gluconeogenesis or heme synthesis 1. Sources of Acetyl CoA – generated directly from B-oxidation of fatty acids and degradation of ketone bodies B-hydroxybutyrate and acetoacetate -also formed from acetate from diet or ethanol oxidation -glucose is oxidized to pyruvate; oxidized to acetyl-CoA by pyruvate dehydrogenase complex -leucine and isoleucine oxidized to acetyl-CoA 2. Pyruvate Dehydrogenase Complex – PDC oxidizes pyruvate to acetyl CoA linking glycolysis and TCA cycle -Structure of PDC – belones to a-keto acid dehydrogenase complex family and shares characteristics, contains E1 (TPP), E2 (lipoate) and E3 (FAD) -E1 and E2 specific to pyruvate, but E3 has an E3 binding protein additionally 3. Regulation of PDC – controlled by phosphorylation of pyruvate dehydrogenase kinase, which inhibits the enzyme and dephosphorylation by pyruvate dehydrogenase phosphatase, which activates it -phosphorylation can decrease activity by 99% -PDC kinase is itself inhibited by ADP and pyruvate, thus when ATP utilization results in increase in pyruvate levels, PDC kinase is inhibited and PDC remains active -PDC requires Ca2+ for full activity -PDC also regulated through product inhibition by Ac-CoA and NADH, because binding of these stimulates its phosphorylation to inactive form -substrates, CoASH and NAD+ antagonize product inhibition -PDC can be activated through insulin mechanism in adipocytes -deficiencies in PDC are common inherited diseases leading to lactic academia and are grouped into category of Leigh disease (acute necrotizing encephalopathy). Most common defects are in gene for a-subunit of E1 (X-linked), and is a problem even if female is a carrier TCA Cycle Intermediates and Anaplerotic Reactions 1. TCA Cycle Intermediates are Precursors for Biosynthetic Pathways – TCA in liver is called ‘open cycle’ because there is high efflux of intermediates -citrate efflux and cleavage to acetyl-CoA provides units for cytosolic fatty acid synthesis -malate leaves mitochondria for gluconeogenesis during fasting -Oxaloacetate and a-ketoglutarate can be used for amino acid synthesis -succinyl coA can go on to Heme synthesis -a-ketoglutarate can be used for neurotransmitters such as GABA or glutamine 2. Anaplerotic Reactions – removal of any of the intermediates from TCA removes 4 carbons that are used to regenerate oxaloacetate during each turn. Without it, TCA cannot continue. Cells supply 4 carbon intermediates from degradation of carbohydrates or amino acids to compensate for rate of removal of oxaloacetate, called Anaplerotic Reactions -Pyruvate Carboxylase is a Major Anaplerotic Enzyme – catalyes addition of CO2 to pyruvate to form oxaloacetate; contains biotin, which forms intermediate with CO2 that requires ATp and Mg -found in liver, brain, adipocytes, and fibroblasts where function is anaplerotic -activated by acetyl-COA and inhibited by acyl-CoA derivatives -Amino Acid Degradation forms TCA Cycle Intermediates – alanine and serin carbons can enter through pyruvate carboxylase. Oxidation of branched chain amino acids such as isoleucine and valine to succinyl-CoA forms an anaplerotic route -compounds that form propionyl CoA (methionine, threonine) can enter TCA as succinyl CoA -Glutamine can be taken up from blood, converted to glutamate, and oxidized to aketoglutarate for another anaplerotic route -TCA cannot be supplied by even-chain length fatty acid oxidation or by ketone body oxidation, which only forms acetyl-CoA Beriberi – a disease known to be caused by thiamine deficiency