The Chemical Logic of TCA cycle
advertisement

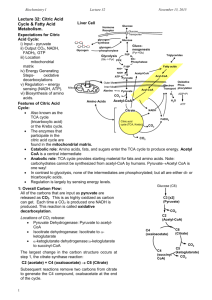
Chapter 19 The Tricarboxylic Acid Cycle Biochemistry by Reginald Garrett and Charles Grisham Essential Question How is pyruvate oxidized under aerobic conditions Pyruvate from glycolysis is converted to acetyl-CoA and oxidized to CO2 in the tricarboxylic acid (TCA) cycle What is the chemical logic that dictates how this process occurs? Hans Krebs showed that the oxidation of acetate is accomplished by a cycle TCA cycle, Citric Acid Cycle or Krebs Cycle • Pyruvate from glycolysis is oxidatively decarboxylated to acetate and then degraded to CO2 in TCA cycle • Some ATP is produced • More NADH and FADH2 are made (24 electrons) • NADH and FADH2 go on to make more ATP in electron transport and oxidative phosphorylation (chapter20) Figure 19.1 (a) Pyruvate produced in glycolysis is oxidized in (b) the tricarboxylic acid (TCA) cycle. (c) Electrons liberated in this oxidation flow through the electron-transport chain and drive the synthesis of ATP in oxidative phosphorylation. In eukaryotic cells, this overall process occurs in mitochondria. 19.1 – What Is the Chemical Logic of the TCA Cycle? • TCA cycle seems like a complicated way to oxidize acetate units to CO2 • Normal ways to cleave C-C bonds in biological systems: 1. cleavage between Carbons and to a carbonyl group (-cleavage) O (fructose bisphosphate aldolase) — C—C— C— 2. -cleavage of an -hydroxyketone (transketolase; fig 22.31) O OH — C—C— The Chemical Logic of TCA cycle • Neither of these cleavage strategies is suitable for acetate • Living things have evolved the clever chemistry of condensing acetate with oxaloacetate and carry out a -cleavage. • TCA combines this -cleavage reaction with oxidation to form CO2, regenerate oxaloacetate and capture all the energy in NADH and ATP Figure 19.2 The tricarboxylic acid cycle. 19.2 – How Is Pyruvate Oxidatively Decarboxylated to Acetyl-CoA? • Pyruvate must enter the mitochondria to enter the TCA cycle • Oxidative decarboxylation of pyruvate is catalyzed by the pyruvate dehyrogenase complex Pyruvate + CoA + NAD+ → acetyl-CoA + CO2 + NADH + H+ • Pyruvate dehydrogenase complex is a noncovalent assembly of three enzymes • Five coenzymes are required Pyruvate dehydrogenase complex (PDC): • Three enzymes and five coenzymes: E1: pyruvate dehydrogenase (24) thiamine pyrophosphate E2: dihydrolipoyl transacetylase (24) lipoic acid E3: dihydrolipoyl dehydrogenase (12) FAD NAD+ CoA • The product of the first enzyme is pass directly to the secondenzyme (a) Domain structure of E2 and E3BP subunits (b) a truncated version of E2 L1 L2 E1BD IC L3 E3BD 30 E1 & 12 E3 (c) Model of the E2/E3BP:E3 core complex (6 E3BP dimer & 48 E2) (d) Model of human PDC Figure 19.3 Models of human pyruvate dehydrogenase Figure 19.4 The reaction mechanism of the pyruvate dehydrogenase complex (TPP) Figure 19.5 The mechanism of the first three steps of the pyruvate dehydrogenase complex reaction The Coenzymes of the Pyruvate Dehydrogenase Complex Thiamine pyrophosphate (vitamin B1 analog) TPP assists in the decarboxylation of α-keto acids (here) and in the formation and cleavage of α-hydroxy ketones (as in the transketolase reaction; see Chapter 22). The Nicotinamide Coenzymes (vitamin B3, niacin analog) NAD+/NADH and NADP+/NADPH carry out hydride (H:-) transfer reactions. All reactions involving these coenzymes are two-electron transfers. The Flavin Coenzymes (vitamin B2) FAD/FADH2 Flavin coenzymes can exist in any of three oxidation states, and this allows flavin coenzymes to participate in one-electron and twoelectron transfer reactions. Partly because of this, flavoproteins catalyze many reactions in biological systems and work with many electron donors and acceptors. Coenzyme A (vitamin B5, pantothenic acid) The two main functions of Co A are: 1. Activation of acyl groups for transfer by nucleophilic attack 2. Activation of the α-hydrogen of the acyl group for abstraction as a proton • The reactive sulfhydryl group on CoA mediates both of these functions. • The sulfhydryl group forms thioester linkages with acyl groups. • The two main functions of CoA are illustrated in the citrate synthase reaction (see Figure 19.6). Lipoic Acid 1. Lipoic Acid functions to couple acyl-group transfer and electron transfer during oxidation and decarboxylation of α-keto acids. 2. It is found in pyruvate dehydrogenase and α-ketoglutarate dehydrogenase. 3. Lipoic acid is covalently bound to relevant enzymes through amide bond formation with the ε-NH2 group of a lysine side chain. 19.3 – How Are Two CO2 Molecules Produced from Acetyl-CoA? Tricarboxylic acid cycle, Citric acid cycle, and Krebs cycle • Pyruvate is oxidatively decarboxylated to form acetyl-CoA Citrate (6C)→ Isocitrate (6C)→ -Ketoglutarate (5C) → Succinyl-CoA (4C) → Succinate (4C) → Fumarate (4C) → Malate (4C) → Oxaloacetate (4C) 1. Citrate synthase reaction • Acetyl-CoA reacts with oxaloacetate in a Perkin condensation (A carbon-carbon condensation between a ketone or aldehyde and an ester) Figure 19.6 Citrate is formed in the citrate synthase reaction from oxaloacetate and acetyl-CoA. The mechanism involves nucleophilic attack by the carbanion of acetyl-CoA on the carbonyl carbon of oxaloacetate, followed by thioester hydrolysis. • Citrate synthase – is a dimer – NADH & succinyl-CoA are allosteric inhibitors • Large, negative G -irreversible Figure 19.7 Citrate synthase in mammals is a dimer of 49-kD subunits. In the monomer shown here, citrate (blue) and CoA (red) bind to the active site, which lies in a cleft between two domains and is surrounded mainly by α-helical segments. 2. Citrate Is Isomerized by Aconitase to Form Isocitrate • Citrate is a poor substrate for oxidation because it contains a tertiary alcohol • So aconitase isomerizes citrate to yield isocitrate which has a secondary -OH, which can be oxidized • Note the stereochemistry of the reaction: aconitase removes the pro-R H of the pro-R arm of citrate • Aconitase uses an iron-sulfur cluster (Fig. 19.9) Aconitase Utilizes an Iron-Sulfur Cluster • Fluoroacetate is an extremely poisonous agent that blocks the TCA cycle • Rodent poison: LD50 is 0.2 mg/kg body weight • Aconitase inhibitor 3. Isocitrate Dehydrogenase Catalyzes the First Oxidative Decarboxylation in the Cycle • Catalyzes the first oxidative decarboxylation in the cycle 1. Oxidation of C-2 alcohol of isocitrate with concomitant reduction of NAD+ to NADH 2. followed by a -decarboxylation reaction that expels the central carboxyl group as CO2 Isocitrate Dehydrogenase • • Isocitrate dehydrogenase links the TCA cycle and electron transport pathway because it makes NADH Isocitrate dehydrogenase is a regulation reaction – – • NADH and ATP are allosteric inhibitor ADP acts as an allosteric activator -ketoglutarate is also a crucial -keto acid for aminotransferase reactions (Chapter 25), connecting the TCA cycle (carbon metabolism) with nitrogen metabolism 4. -Ketoglutarate Dehydrogenase • • Catalyzes the second oxidative decarboxylation of the TCA cycle This enzyme is nearly identical to pyruvate dehydrogenase - structurally and mechanistically 1. -ketoglutarate dehydrogenase 2. Dihydrolipoyl transsuccinylase 3. Dihydrolipoyl dehydrogenase (identical to PDC) • Five coenzymes used - TPP, CoA-SH, Lipoic acid, NAD+, FAD Like pyruvate dehydrogenase, -ketoglutarate dehydrogenase is a multienzyme complex – consisting of -ketoglutarate dehydrogenase, dihydrolipoyl transsuccinylase, and dihydrolipoyl dehydrogenase. The complex uses five different coenzymes. 19.4 – How Is Oxaloacetate Regenerated to Complete the TCA Cycle? 5. Succinyl-CoA Synthetase A substrate-level phosphorylation GTP + ADP ATP + GDP (nucleotide diphosphate kinase) • A nucleoside triphosphate is made Thioester • Its synthesis is driven by hydrolysis of a CoA ester [Succinyl-P] • The mechanism involves a phosphohistidine [Phosphohistidine] GTP Figure 19.11 The mechanism of the succinyl-CoA synthetase reaction. Completion of the TCA Cycle – Oxidation of Succinate to Oxaloacetate • This process involves a series of three reactions • These reactions include: 1. Oxidation of a single bond to a double bond (FAD/FADH2) 2. Hydration across the double bond 3. Oxidation of the resulting alcohol to a ketone (NAD+/NADH) • These reactions will be seen again in oxidation of fatty acids 6. Succinate Dehydrogenase • The oxidation of succinate to fumarate • A membrane-bound enzyme is actually part of the electron transport chain in the inner mitochondrial membrane (succinate-CoQ reductase) • The reaction is not sufficiently exergonic to reduce NAD+ (trans-) • The electrons transferred from succinate to FAD (to form FADH2) are passed directly to ubiquinone (UQ) in the electron transport pathway (chapter 20) • FAD is covalently bound to the enzyme • Contains iron-sulfur cluster Succinate Dehydrogenase contains three types of ironsulfur clusters: a 4Fe-4S cluster, a 3Fe-4S cluster, and a 2Fe-2S cluster. Figure 19.12 The covalent bond between FAD and succinate dehydrogenase links the C-8a carbon of FAD and the N-3 of a His residue of the enzyme. 7. Fumarase Hydration across the double bond • Catalyzes the trans-hydration of fumarate to form L-malate • trans-addition of the elements of water across the double bond • Possible mechanisms are shown in Figure 19.13 8. Malate Dehydrogenase • Completes the Cycle by Oxidizing L-Malate to Oxaloacetate • This reaction is very endergonic, with a Go' of +30 kJ/mol 19.5 – What Are the Energetic Consequences of the TCA Cycle? One acetate through the cycle produces two CO2, one ATP, four reduced coenzymes Acetyl-CoA + 3 NAD+ + FAD + ADP + Pi + 2 H2O → 2 CO2 + 3 NADH + 3 H+ + FADH2 + ATP + CoASH G0’ = -40kJ/mol Glucose + 10 NAD+ + 2 FAD + 4 ADP + 4 Pi + 2 H2O → 6 CO2 + 10 NADH + 10 H+ + 2 FADH2 + 4 ATP NADH + H+ + 1/2 O2 + 3 ADP + 3 Pi → NAD+ + 3ATP + H2O FADH2 + 1/2 O2 + 2 ADP + 2 Pi → FAD + 2ATP + H2O The Carbon Atoms of Acetyl-CoA Have Different Fates in the TCA Cycle Neither of the carbon atoms of a labeled acetate unit is lost as CO2 in the first turn of the cycle 1.Carbonyl C of acetyl-CoA turns to CO2 only in the second turn of the cycle (following entry of acetyl-CoA ) 2.Methyl C of acetyl-CoA survives two cycles completely, but half of what's left exits the cycle on each turn after that. The Carbon Atoms of Acetyl-CoA Have Different Fates in the TCA Cycle Figure 19.15 The fate of the carbon atoms of acetate in successive TCA cycles. (a) The carbonyl carbon of acetyl-CoA is fully retained through one turn of the cycle but is lost completely in a second turn of the cycle. 19.6 – Can the TCA Cycle Provide Intermediates for Biosynthesis? The products in TCA cycle also fuel a variety of biosynthetic processes • α-Ketoglutarate is transaminated to make glutamate, which can be used to make purine nucleotides, Arg and Pro • Succinyl-CoA can be used to make porphyrins • Fumarate and oxaloacetate can be used to make several amino acids and also pyrimidine nucleotides Figure 19.16 The TCA cycle provides intermediates for numerous biosynthetic processes in the cell. • Citrate can be exported from the mitochondria and then broken down by citric lyase to yield acetyl-CoA and oxaloacetate (chapter 24) • Oxaloacetate is rapidly reduced to malate • Malate can be transported into mitochondria or oxidatively decarboxylated to pyruvate by malic enzyme • Oxaloacetate can also be decarboxylated to yield phosphoenolpyruvate 19.7 – What Are the Anaplerotic, or “Filling Up,” Reactions? • Pyruvate carboxylase - converts pyruvate to oxaloacetate (in animals), is activated by acetyl-CoA (chapter 22, gluconeogenesis) • PEP carboxylase - converts PEP to oxaloacetate (in bacteria & plants), inhibited by aspartate • Malic enzyme converts pyruvate into malate • The catabolism of amino acids provides pyruvate, acetyl-CoA, oxaloacetate, fumarate, -ketoglutarate, and succinate (chapter 25). • PEP carboxykinase – Could have been an anaplerotic reaction. – CO2 binds weakly to the enzyme, whereas oxaloacetate binds tightly – The reaction favors formation of PEP from oxaloacetate 19.8 – How Is the TCA Cycle Regulated? 1. Citrate synthase – ATP, NADH and succinyl-CoA inhibit 2. Isocitrate dehydrogenase – ATP and NADH inhibits – ADP and NAD+ activate 3. -Ketoglutarate dehydrogenase – NADH and succinyl-CoA inhibit – AMP activates 4. Pyruvate dehydrogenase – ATP, NADH, acetyl-CoA inhibit – NAD+, CoA activate Regulation of the TCA cycle. 19.8 – How Is the TCA Cycle Regulated? When the ADP/ATP or NAD+/NADH ratio is high, the TCA cycle is turned on Succinyl-CoA is an intracycle regulator, inhibiting citrate synthase and ketoglutarate dehydrogenase Acetyl-CoA acts as a signal to the TCA cycle that glycolysis and fatty acid breakdown is producing two-carbon unit 1. Activate pyruvate carboxylase 2. Feedback inhibit pyruvate dehydrogenase Pyruvate dehydrogenase is regulated by phosphorylation/dephosphorylation Animals cannot synthesize glucose from acetylCoA, so pyruvate dehydrogenase complex plays a pivotal role in metabolism • Allosterically regulation – Inhibit by Acetyl-CoA (dihydrolipoyl transacetylase), or NADH (dihydrolipoyl dehydrogenase) • Covalently modification on pyruvate dehydrogenase – Phosphorylation (pyruvate dehydrogenase kinase) – Dephosphorylation (pyruvate dehydrogenase phosphatase) 1. The pyruvate dehydrogenase kinase (PDK; Fig 19.3) is associated with the enzyme – Allosterically activated by NADH and acetyl-CoA – Phosphorylated pyruvate dehydrogenase subunit is inactive 2. Reactivation of the enzyme by pyruvate dehydrogenase phosphatase – A Ca2+-activated enzyme – Hydrolyzes the phosphoserine moiety on the dehydrogenase subunit – Insulin and Ca2+ activate dephosphorylation – Pyruvate inhibit dephosphorylation 19.9 – Can Any Organisms Use Acetate as Their Sole Carbon Source? • Plant and some bacteria can use acetate as the only source of carbon for all the carbon compounds • plants and some bacteria employ a modification of the TCA cycle called the glyoxylate cycle to produce four-carbon compounds from acetylCoA • The CO2-producting steps are bypassed and an extra acetate is utilized • Isocitrate lyase and malate synthase are the short-circuiting enzymes (Fig 19.21) Figure 19.21 The glyoxylate cycle. Glyoxylate Cycle In plants, the glyoxylate cycle is carried out in glyoxysomes, but yeast and algae carry out in cytoplasm 1.Isocitrate lyase – produces glyoxylate and succinate – Is similar to the aldolase reaction in glycolysis 2.Malate synthase – A Claisen condensation of acetyl-CoA and the aldehyde group of glyoxylate to form L-malate – Is similar to the citrate synthase reaction Figure 19.22 The isocitrate lyase reaction. • The glyoxylate cycle helps plants grow in the dark – – – • Certain seeds grow underground, where photosynthesis is impossible Many seeds are rich in lipids Once the growing plant begins photosynthesis and can fix CO2 to produce carbohydrate, the glyoxysomes disappear Glyoxysomes must borrow three reactions from mitochondria: succinate to oxaloacetate 1. Succinate dehydrogenase 2. Fumarate 3. Malate dehydrogenase Figure 19.23 Glyoxysomes lack three of the enzymes needed to run the glyoxylate cycle. Succinate dehydrogenase, fumarase, and malate dehydrogenase are all “borrowed” from the mitochondria in a shuttle in which succinate and glutamate are passed to the mitochondria, and α-ketoglutarate and aspartate are passed to the glyoxysome.