Alcohol Dehydrogenase
advertisement

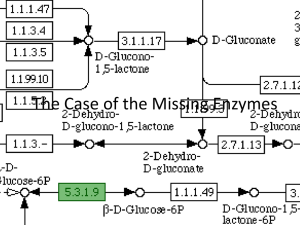
ALCOHOL DEHYDROGENASE A comprehensive overview and experimental analysis Elliott Weideman ALCOHOL DEHYDROGENASE What is it? ADH Enzyme that breaks down alcohols Why is it important? In most animals it is used to metabolize alcohols that have been ingested. Yeasts and bacteria use ADH in reverse to convert sugars into ethanol via fermentation. ALCOHOL DEHYDROGENASE Chemical Reaction RCH2OH + NAD+ RCHO + NADH + H+ NAD+ is needed as an important cofactor for ADH ADH ALCOHOL DEHYDROGENASE Experimental Question What is the rate of reaction that ADH is capable of maintaining? Experimental Design Overview Chemical assay techniques using spectrophotometry to measure the amount of product synthesized from ADH. ALCOHOL DEHYDROGENASE Materials S. Cerevisiae Yeast ADH - Provided from Sigma NAD+ - Provided from Sigma 95% Ethanol - Provided from Sigma Tris Buffer pH 8 - Provided from Acros Equipment Genesys 10 UV/vis Spectrophotometer - Provided from ThermoSpectronic Cuvettes - Provided from Fisher Scientific ALCOHOL DEHYDROGENASE Procedure Obtain Tris buffer - pH 8 Prepare ADH enzyme by adding dry reagent with water to equal 20 units/mL Dilute Ethanol to concentrations of 0.7M, 0.375M, 0.1M, 0.5M and 0.25M C1 V1 =C2 V2 Prepare NAD+ to final concentration of 15mM ALCOHOL DEHYDROGENASE Procedure Enzyme assays by combining the following 900uL Tris buffer 33uL NAD+ 33uL ethanol (0.7M, 0.375M, 0.1M, 0.05M, and 0.025M) 33uL ADH The solution was mixed thoroughly and gently before quickly being measured in the spectrophotometer at 340nm ALCOHOL DEHYDROGENASE Procedure Assays were measured for absorbance at T0 and T1 Average rate (Abs340 T1 – Abs340T0/min) was recorded Three trials were run for each [ethanol] ALCOHOL DEHYDROGENASE [Ethanol] Data M Abs T0 Abs T1 0.7 0.375 0.1 0.05 0.025 3+ 2.923 2.917 2.466 1.072 1.946 1.066 1.215 1.283 0.904 0.854 0.697 0.651 0.566 0.636 3+ 3+ 3+ 3+ 1.102 3+ 2.182 2.229 2.163 1.443 1.47 1.197 1.152 0.928 1.077 Rate (ΔA340 /min) Average Rate 0.080+ 0.083+ 0.534+ 0.030 1.054+ 1.116 1.014 0.880 0.539 0.616 0.500 0.501 0.362 0.441 0.082+ 0.539+ 1.003 0.552 0.435 ALCOHOL DEHYDROGENASE Results 1.8 y = 0.0277x + 0.5428 Lineweaver-Burke Data and Plot R² = 0.9938 1.6 1/Absorbance 340nm 1/[Ethanol] 1.4 1/Abs T1 1.4285 0.539539 1.2 2.6667 0.617408 1 10 0.84685 0.8 20 1.13729 0.6 40 1.62507 0.4 0.2 0 -30 -20 -10 -0.2 0 10 1/[Ethanol] M 20 30 40 50 ALCOHOL DEHYDROGENASE Results Vmax – The fastest the enzyme can perform under ideal circumstances 0.0511 M/min Km – Michaelis constant (several rate constants) 1.8423 mM 3D rendering of ADH ALCOHOL DEHYDROGENASE Discussion Drawbacks Incorrect preparation of trial 2 assay for 0.375M Ethanol Possible contamination when preparing NAD+ Inconsistent handling of cuvettes when loading into spectrophotometer Uniform mixing Time loading Use less ADH enzyme to get a more accurate reading of the rate of reaction Positive control – Lacking an inhibited version of the enzyme ALCOHOL DEHYDROGENASE Discussion Future Research Investigate performance of ADH with other concentrations of ethanol under different temperatures or levels of pH Run the reaction backwards by manipulating the equilibrium level Use other versions of ADH and or types of alcohols Applications to medical industry involving alcoholism and genetics ALCOHOL DEHYDROGENASE Discussion Application Cirrhosis of liver – condition marked by chronic liver disease and subsequent degeneration ALCOHOL DEHYDROGENASE References Bendinskas, K., DiJiacomo, C., Krill, A., & Vitz, E. 2005. Kinetics of alcohol dehydrogenase – catalyzed oxidation of ethanol followed by visible spectroscopy, Journal of Chemical Education, 82(7), 1068-1070. Edenberg, H. J. 2007. The genetics of alcohol metabolism: Role of alcohol dehydrogenase and aldehyde dehydrogenase variants. Alcohol Research & Health, 30(1), 5-13. Voss, C. Gruber, K. Faber, T. Knaus, P. Macheroux, W. Kroutil. 2008. J. Am. Chem. Soc, 130, 13969-13972. Laboratory Manual, 2012. Dr. Christenson