Spana-Final Deliverable - the Biology Scholars Program Wiki
advertisement

Final Deliverable
2013 Assessment residency
Eric Spana
The week I have chosen to work out is week 2 (the second & third classes) of
Biology 414LS, Experiments in Genetics and Development. In this course, which
meets from 1:25-5:25 on Tuesdays and Thursdays, the students (typically 12) are
broken up into 4 teams. Each team is given a Drosophila mutation that has a
recessive wing morphology phenotype. The mutation has had some mapping done
(typically in the early to mid 20th century) but the underlying gene/transcription
unit that is affected is unknown.
On the first day of class, students are picked schoolyard style into teams and
assigned their mutants. They are given the links to the mutants on the Drosophila
database FlyBase (www.flybase.org) and the students spend the day looking at their
mutant flies and wild-type flies and given a brief “how to use the microscope in front
of you” lesson. We adjourn early.
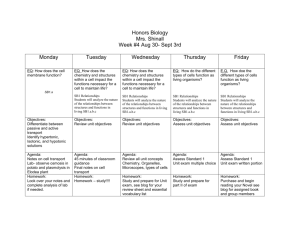
Week 2 Day 1 (Tuesday):
A PowerPoint lecture on “Normal Fly Development & Microscopy” is given
during first part of class. Reading for this class period includes “Tyler Lab Manual
Ch8-Drosophila” and “Drosophila Guide” Introduction by Demerec & Kaufman.
A brief outline of the lecture:
1. Embryonic Development
2. Post-embryonic timing
3. 3rd instar larval “structure”
4. 3rd instar imaginal discs form adult structures
5. Wing imaginal disc fate map
6. Adult wing parts
Activity: How to dissect imaginal discs
Following this lecture is an activity: dissecting imaginal discs from 3rd instar larvae.
The protocol is written on the board (so they have to write it in their notebooks)
and they attempt to do the dissections they have just seen in the PowerPoint movies
and instructor demonstration.
The lecture and activity are to achieve these objectives:
*Students will be able to briefly describe how a fertilized egg will develop into a 1 st
instar larvae with regard to patterning, germ layers & germ cells.
*Students will be able to describe the life cycle with regard to timing of stages.
*Students will be able to identify the parts of an adult wing.
*Students will be able to dissect and identify larval imaginal discs by shape and
position.
Other goals for the day include working through an entire 4 hour class period,
finding the reagents in the lab, writing in their notebooks, pipetting, failing
miserably, getting messy, etc.
Week 2 Day 2 (Thursday):
A PowerPoint lecture on “Fly Genetics, Husbandry & Handling” is given.
Reading for this class period is “Fly Pushing, 2nd Edition, Chapter 1 by Greenspan. A
brief outline of the lecture:
Life cycle review
Dmel chromosome content
Chromosome cytological map
Chromosome genetic map
Chromosome nucleotide sequence
Gene nomenclature & how to write genotypes
Dmel dosage compensation
Male recombination
How balancers work
How to identify males & females
How to identify virgins
How to use dissecting microscopes & CO2
Activity: Sorting males & females, virgins
Following the lecture is an activity: sorting males & females and identifying virgins.
It is of utmost importance that they can do this every single time, because they will
get false positives (or false negatives) if they pick fertilized females instead of virgin
females. The instructor will check how they did, and students can and will do this
with multiple samples and/or genotypes.
The lecture and activity are to achieve these objectives:
*Students will be able to explain the relationship between genome sequence, genetic
& cytological maps across the chromosome.
*Students will be able to predict (in a very, very rough way) where their mutation
lies on the genome by comparing the known genetic and/or cytological map
position for their gene.
*Students will be able to describe what an adult fly would look like given a genotype.
*Students will be able to explain the differences between Drosophila and human
dosage compensation
*Students will be able to explain what balancers are used for and which is used for
which chromosome.
*Students will be able to sort and show the difference between sexes & virgins.
*Students will be able to set up the light source & dissecting microscope.
The class will then have their first assessment: Sabotage! The last objective (setting
up their microscope correctly) is important as the set up for fly work is very
different from that of dissections. They need to be able to go back and forth so that
they can visualize individual tissues (in dissections) and visualize subtle genetic
markers on adult flies. The assessment is graded for completion as part of the
course “Class Participation” grade. (Or as I refer to it: Being the leader, professional
and scientist that your CV says you are.)
Name____________________________
http://www.youtube.com/watch?v=z5rRZdiu1UE
Take a sample of the dead flies being distributed. We don’t need to waste CO2 on
keeping flies asleep for this exercise, so we will just use dead ones.
Set up your microscope and light source the best you can to focus the fly’s wings.
Note the magnification knob setting:______________________
Note the ocular settings: L______________________________
R______________________________
Note the intra-ocular distance:______________________
Nice job.
Now take your team to the microscopes in the same position on the other bench and
look at ALL THREE microscopes set up there.
How is the view of the subject through each of the three microscopes WITHOUT
ADJUSTING ANYTHING?
Rate the three microscopes: BEST, MIDDLE, WORST with regard to how well you
can resolve detail on the fly’s wings.
BEST:______________________
MIDDLE:______________________
WORST:______________________
Now fiddle with the microscope and/or light to make it better. What did you
change?
Now modify the settings on the other team’s microscopes. Make it as off as you can,
but DO NOT REMOVE ANY PARTS!!! Change each microscope in a different way—
the settings should not be identical.
What settings did you change?
Congratulations! You have sabotaged the other team!
Now go back to your microscope. What settings do you think were changed?
Which microscope is worst/ who did the best job of sabotage?
Now fix it! Put it back as best you can. If you get stuck and things just don’t seem
right, concede defeat and go crawling to the saboteur and beg for their help. If
someone comes for help, be the kind generous leader your CV says you are and help
them to put the microscope back to optimal settings. Then go into the hallway and
fist-pump like crazy. You win.
Summative Assessment:
Big course objective to match:
Students will be able to design an experiment to identify a mutation by
mapping techniques and illustrate how that mutation in the genome could affect a
protein and how that change could cause a phenotype. (Synthesis & Application)
Daily course objective to match from week 2:
Students will be able to explain the relationship between sequence, genetic &
cytological maps across the chromosome.
Students will be able to describe what an adult fly would look like given a
genotype.
Question from Quiz 1 that happens after week 3. The quiz is take home, and open to
everything except other people. It’s a research question, where the student is
expected to look up the genotypes perform the cross on paper, and describe what
the phenotypes of the resulting progeny look like. It prepares them for
understanding what to expect when they do the crosses that they proposed during
week 3 (there are multiple genetic markers in these stocks, and most crosses they
do will actually have slightly different results). In week 2 we discussed the
connection between sequence, genetic and cytological maps and in week 3 we
discussed molecularly defined deficiency strains and complementation.
E. (8 pts) Write out the progeny of the crosses for virgin females of one of the
deficiencies above crossed to males of Bloomington stock 1686 (D1 red1 e1/TM3, Sb1)
and describe what the phenotype of males and females of each of the progeny
classes would be regarding whether they have a red tubule phenotype, eye color,
wing morphology, bristle morphology, body color etc. assuming red DOES lie within
the breakpoints of the deletions.
Answer key:
For virgins of BL9279 (w1118; Df(3R)ED5642, P{3'.RS5+3.3'}ED5642/TM6C, cu1 Sb1) x males of
BL1686, the cross would yield:
Males: w1118/ㄱ; D1 red1 e1/TM6C e1 cu1 Sb1: white eyes, they have Dichaete wings (wings held out),
ebony body color (since ebony is on both TM6C and the red stock), and Stubble bristles.
Females would be w1118/+ so would have wild-type colored eyes and the rest would be the same.
Males and females of TM6C/TM3 are dead, because Sb is homozygous lethal.
Males of w1118/ㄱ; Df(3R)ED5642 / TM6, e1 cu1 Sb1: mini-white eyes (the deletion creates a functional
mini white gene). This male also has Stubble bristles. Females would be w1118/+ so they would have wildtype colored eyes.
Males of w1118/ㄱ; Df (3R) ED5642 / D1 red1 e1 have mini-white eyes, so can’t detect any red phenotype.
Dichaete wings. Females would be w1118/+, so they would have red mutant eye color.
The way this cross was set up, you can only score the red gene in the females, since they will be
heterozygous for white. Many of the deficiencies have mini-white at the site of the deletion, so some will
be mini-white+, and some will not.
A Multiple Response version:
In a cross between stock BL#9279 (w1118; Df(3R)ED5642,
P{3'.RS5+3.3'}ED5642/TM6C, cu1 Sb1) and BL#1686 (D1 red1 e1/TM3, Sb1) which of
the following progeny would be found? Mark all correct answers.
A.
B.
C.
D.
E.
a male with a red phenotype
a female with a red phenotype
a white eyed male with Stubble bristles
a white eyed female with Stubble bristles
a white eyed male with a black body color
A short answer version:
In a cross between stock BL#9279 (w1118; Df(3R)ED5642,
P{3'.RS5+3.3'}ED5642/TM6C, cu1 Sb1) and BL#1686 (D1 red1 e1/TM3, Sb1) briefly
describe whether you could get a fly that looks like the one described and what its
genotype would be:
A. A male with a red phenotype
B. A female with a red phenotype
C. A white eyed male with Stubble bristles
D. A white eyed female with Stubble bristles
E. A white eyed male with a black body color