LIPOPROTEIN LIPASE DEFICIENCY, HYPERTRIGLYCERIDEMIA
advertisement

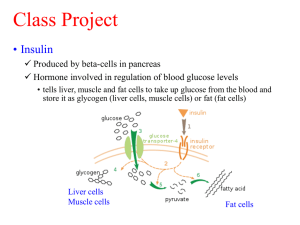
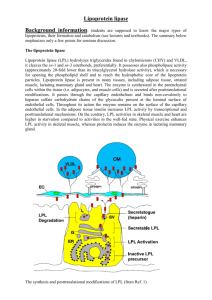
LIPOPROTEIN LIPASE DEFICIENCY, HYPERTRIGLYCERIDEMIA, AND INSULIN RESISTANCE PhD student Sofia Beck Mikkelsen, Department of Endocrinology M, Odense University Hospital The metabolic syndrome is comprised of a cluster of metabolic disorders, of which many promote the development of atherosclerosis, increase the risk of cardiovascular disease and diabetes. Insulin resistance is at the heart of the metabolic syndrome. Elevated serum triglycerides commonly associate with insulin resistance and represent a valuable clinical marker of the metabolic syndrome. Familial lipoprotein lipase (LPL) deficiency is an inherited condition that disrupts the normal breakdown of triglycerides in the body. It is increasingly being recognized that heterozygous mutations that cause decreased LPL activity are associated with insulin resistance and the metabolic syndrome in humans, however, the molecular mechanisms underlying this association is not known. We hypothesize that mutations within the lipoprotein lipase gene lead to accumulation of lipid metabolites in skeletal muscle that impairs insulin signalling and glucose metabolism which subsequently induce insulin resistance. By means of molecular biology and biochemistry we aim to identify the molecular mechanisms by which hypertriglyceridemia induces insulin resistance in skeletal muscle in human subjects. Blood samples from hypertriglyceridemic subjects with and without Type 2 Diabetes are used to identify novel mutations within the LPL gene or within regulators of LPL activity. Whereas blood samples and muscle biopsies taken from patients with hypertriglyceridemia carrying a well-defined LPL mutation and healthy subjects is used to identify and quantify alterations in the lipid composition of plasma and muscle cells, and to examine how severe hypertriglyceridemia (induced by LPL mutations) affects insulin sensitivity, glucose and fat metabolism, and insulin signalling in skeletal muscle in vivo. Novel LPL mutations are identified by DHPLC, and candidates will be selected and identified by DNA sequencing. Lipidomic profiling in response to loss of lipoprotein lipase activity is determined by lipid mass spectrometry, and the overall fatty acid- and acyl-coA composition is examined by gas chromatography and HPLC, respectively. Genes previously described to be regulated by insulin and possible regulatory genes will be examined by qRT-PCR. The level and phosphorylation of protein components involved in insulin signalling including the insulin receptor, IRS proteins, Akt1/PKB, GSK3, and FOXOs will be examined by Western Blotting. Based on the results obtained from the qRT-PCR and Western blotting analyses, mechanistic studies in cultured muscle cells are performed.





![Lipolysis[1] - IHMC Public Cmaps](http://s2.studylib.net/store/data/005358142_1-87bcfc0fc3c32a571191c55d07580764-300x300.png)