Assg: NOTEBOOK Bozeman Water: A Polar Molecule Answer the
advertisement

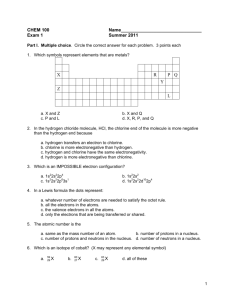
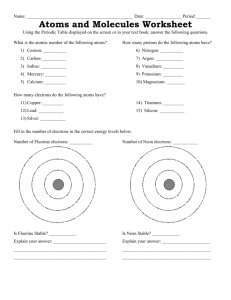
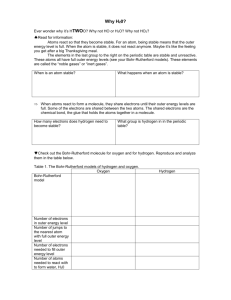
Assg: NOTEBOOK Bozeman Water: A Polar Molecule Answer the following questions in your notebook. Remember to watch this video you go to our school website, Henriques webpage, and in the upper right click on the video tab. Find the video titled “Bozeman Water: A Polar Molecule”. Answer all questions in your NOTEBOOK. Staple this paper somewhere in the notebook so you know the questions to all the answers you wrote in your notebook. 1. Where you find you find life! 2. PAUSE AND ANSWER QUESTIONS 3-9 3. Oxygen’s atomic number is 8. Draw an oxygen atom with the protons, neutrons and electrons (be sure to place the correct number of electrons in each shell). 4. Hydrogen’s atomic number is 1. Draw an oxygen atom with the protons and electrons (be sure to place the correct number of electrons in each shell). 5. Looking at your drawing, how many electrons does oxygen need to become stable? 6. Looking at your drawing, how many electrons does hydrogen need to become stable? 7. True or False? If the two molecules your just drew could share electrons, both atoms would be stable. 8. Explain your answer to the previous question. 9. So what can happen next so that both atoms are stable? 10. Play the video but as soon as you see “Oxygen is electronegative” pause and draw the diagram on you see on 11. What does electronegative mean? 12. Which atom is more electronegative? Oxygen or hydrogen? 13. Oxygen has ___ valence electrons. 14. If ionic bonds don’t hold oxygen and hydrogen together, what type of bond does? 15. Draw the water molecule you see on the right. Use this diagram to explain why water is polar. 16. Draw the molecule on the right. Identify all the oxygen atoms and hydrogen atoms. Then identify the charge on all the oxygen atoms and then the charge on all the hydrogen atoms. 17. Circle where hydrogen bonding will occur. 18. Explain what is hydrogen bonding. 19. Water is a polar molecule while fat is a ____ 20. Water is charged therefore it can only grab on to other parts that are _____ 21. Remember the phrase _____ dissolves _____ 22. Explain the property high specific heat. 23. Explain why water is often called the universal solvent. 24. In cohesion water molecules will bind to ____ 25. Why can a paper clip float on water? 26. Explain capillary action. 27. As water gets colder is get ____ dense.