Atomic Spectra - Mountain View College
advertisement

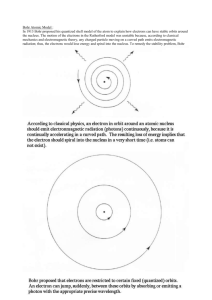
Atomic Spectra Name(s): Course: Date: Section: Theory: The emission of a unique colored light by gaseous elements when subjected to high voltage baffled scientist for a long time unit Neil Bohr came up with the Bohr’s atomic model, which solved this mystery. Bohr model is that electrons spin around the nucleus like planets spin around the sun. Also that the electron orbits are quantized, that is electrons can be in one fixed orbit or another fixed orbit but not between the orbits and that each of the orbits related to a certain energy level of the electron. Lastly, electrons can move from a lower orbit to a higher orbit if it is given energy by an external source such as a high voltage power supply. This energy is given in electron-volts or eV. The orbit that it jumps to will vary depending upon the amount of energy the electron absorbs. The electron will not remain in this orbit but will decay back to its original orbit. This decay may be in a single step or in multiple steps however each time the electron decays to lower orbit energy is loss and a photon is emitted. The wavelength or color of the photon is dependent on the amount of energy that is loss. A spectral line is a dark or bright line in an otherwise uniform and continuous spectrum, resulting from a deficiency or excess of photons in a narrow frequency range, compared with the nearby frequencies, generated by the transition of electrons from higher to lower orbit or vice versa. Spectral lines are often used as a sort of "atomic fingerprint," as gases emit light at very specific frequencies when exposed to electromagnetic waves, which are displayed in the form of spectral lines. These "fingerprints" can be compared to the previously collected fingerprints of elements, and are thus used to identify the molecular construct of stars and planets which would otherwise be impossible. The spectroscope will be used to view the spectra of the elements. At the wider end is a small slit. This slit is where the light emitted from the spectra tube enters into the spectroscope. The narrow end of the spectroscope is the eyepiece thru which you will look into the spectroscope. Procedure: □ While looking into the spectroscope point the large end of the spectroscope towards the light source you should see the narrow slit at the opposite end. □ Move the spectroscope from side to side to locate the spectral source, light from the spectral source should fill the slit. □ Once this is achieved and with the light filling the slit adjust your line of sight towards the right. There you will see a scale and a spectrum, various wavelengths that composes the color of the light source. Take note of where the lines are in relation to the scale. □ Determine what the element is for the spectral tube. Confirm your observations with published Spectral Charts. Substance: Substance: V 400 I 445 B G 475 510 Y 570 O 590 R 650 nm 400 I 445 B G 475 510 Y 570 O 590 R 650 nm 400 I 445 400 445 B G 475 510 Y O 570 R 590 650 nm V 400 I 445 B G 475 510 Y 570 O 590 R 650 nm Substance: Substance: V I Substance: Substance: V V B 475 G 510 Y 570 O 590 R 650 nm V 400 I 445 B G 475 510 Y 570 O 590 R 650 nm