Supplementary Table 2. Overview of observational studies on
advertisement

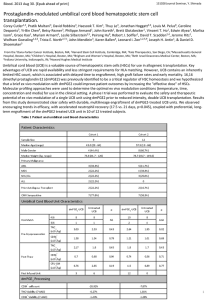
Supplementary Table 2. Overview of observational studies on associations of NSAID exposure in the second and/or third trimester and other pregnancy outcomes Country Study design Exposure Comparison Outcome Data source Size group Main findings Time period USA CC Any NSAID, n = 33 No PPHN PPHN: NS Slone Epidemiology n = 1,213 OTC NSAIDs included (n = 836) Center’s Birth Defects Study (1998–2003) USA CC Any NSAID, n = 64 Unexposed to Quadriparetic CP in children born < 28 weeks after LMP: The ELGAN Study n = 877 OTC NSAIDs included NSAIDs For any NSAID: aOR 2.4; 95% CI 1.04–5.8 Children < 28 weeks old (n = 813) Diaparetic CP in children born < 28 weeks after LMP: in 11 institutions For any NSAID: aOR 3.5; 95% CI 1.1–11.0 (2002–2004) USA CH Indomethacin, n = 85 Unexposed to Grade III or IV IVH in VLBW babies: The NICHD Neonatal n = 573 No OTC NSAIDs indomethacin For indomethacin: aOR 2.7; 95% CI 1.2–6.4 Research Network’s Data (n = 464) Base Study (1995–2000) USA CC Any NSAID, n = 50 No PPHN PPHN: Hospital records, n = 101 Ibuprofen, n = 23 (n = 61) For any NSAID: aOR 21.5; 95% CI 7.1–64.7 meconium analysis Naproxen, n = 19 For ibuprofen: aOR 12.9; 95% CI 3.9–42.3 (2001) Acetylsalicylic acid, n = For naproxen: aOR 3.3; 95% CI 1.2–9.3 44 For acetylsalicylic acid: aOR 8.1; 95% CI 3.3–20.0 OTC NSAIDs included Finland CC Indomethacin, n = 31 No renal Renal damage: NS Record analysis n = 66 No OTC NSAIDs damage at (2001) < 33 gestational weeks (n = 35) Finland CH Indomethacin, n = 82 None Grade I or II IVH: Record analysis n = 240 No OTC NSAIDs For indomethacin dose > 150mg/day: aOR 3.9; 95% CI 1.4–10.7 (2001) For indomethacin therapy > 2 days: aOR 3.4; 95% CI 1.3–8.9 Severe NEC: For indomethacin dose > 150mg/day: aOR 4.3; 95% CI 1.2–14.4, for indomethacin therapy > 2 days: aOR 4.4; 95% CI 1.1–17.4 Sepsis: For indomethacin: aOR 9.3; 95% CI 2.3–36.9 Ref. 1 2 3 4 5 6 USA Record analysis (1991–1995) USA Record analysis (1986– 1991) CH n = 72 Indomethacin, n = 72 No OTC NSAIDs None Premature closure of ductus arteriosus: For indomethacin with advancing gestational age, p < 0.05. 7 CC n = 114 Indomethacin, n = 57 No OTC NSAIDs Unexposed to indomethacin at corresponding gestational age (n = 57) Increased risk of premature closure of ductus arteriosus: For indomethacin: p < 0.05 NEC: For indomethacin: p < 0.005 Grade II or IV IVH: For indomethacin: p < 0.02 Oliguria: For indomethacin: p < 0.003 8 Abbreviations: CH, cohort; CC, case–control; PPHN, persistent pulmonary hypertension of the newborn; NS, no excess risk (statistically not significant); ELGAN, Extremely Low Gestational Age Newborns; CP, cerebral palsy; LMP, last menstrual period; aOR = adjusted OR; IVH = intraventricular haemorrhage; VLBW = very low birth weight (< 1500g); NEC = necrotizing enterocolitis. 1. 2. 3. 4. 5. 6. 7. 8. Van Marter, L.J., Hernandez-Diaz, S., Werler, M.M., Louik, C. & Mitchell, A.A. Nonsteroidal antiinflammatory drugs in late pregnancy and persistent pulmonary hypertension of the newborn. Pediatrics 131, 79-87 (2013). Tyler, C.P. et al. Brain damage in preterm newborns and maternal medication: the ELGAN Study. Am. J. Obstet. Gynecol. 207, 192 e1-9 (2012). Doyle, N.M., Gardner, M.O., Wells, L., Qualls, C. & Papile, L.A. Outcome of very low birth weight infants exposed to antenatal indomethacin for tocolysis. J. Perinatol. 25, 336-340 (2005). Alano, M.A., Ngougmna, E., Ostrea, E.M., Jr. & Konduri, G.G. Analysis of nonsteroidal antiinflammatory drugs in meconium and its relation to persistent pulmonary hypertension of the newborn. Pediatrics 107, 519-523 (2001). Ojala, R. et al. Renal follow up of premature infants with and without perinatal indomethacin exposure. Arch. Dis. Child Fetal. Neonatal Ed. 84, F28-F33 (2001). Ojala, R., Ikonen, S. & Tammela, O. Perinatal indomethacin treatment and neonatal complications in preterm infants. Eur. J. Pediatr. 159, 153-155 (2000). Vermillion, S.T., Scardo, J.A., Lashus, A.G. & Wiles, H.B. The effect of indomethacin tocolysis on fetal ductus arteriosus constriction with advancing gestational age. Am. J. Obstet. Gynecol. 177, 256-259; discussion 259-261 (1997). Norton, M.E., Merrill, J., Cooper, B.A., Kuller, J.A. & Clyman, R.I. Neonatal complications after the administration of indomethacin for preterm labor. N. Engl. J. Med. 329, 1602-1607 (1993).