NSAIDs and Fracture Healing :: Hwang
advertisement

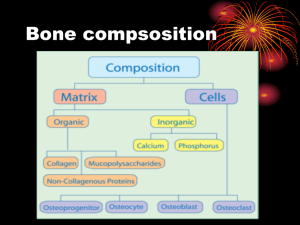
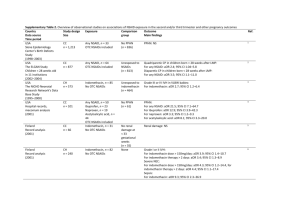
NSAIDs and Fracture Healing by Michael Hwang In a recent review on orthopaedic research by Rodeo et al (1), the authors assert that, “It has been well established that nonsteroidal anti-inflammatory drugs can inhibit bone-healing.” However, contrary to what many believe, the literature does not support such a unanimous conclusion. The first experiments on the effect of NSAIDS on fracture healing occurred in animals. One of the earliest was a randomized controlled trial in rats by Allen et al in 1980 (2). The authors took 210 rats and subjected them to fractures of their right ulna and radius. These rats were then divided into seven groups of 30 and given varying doses of indomethacin and aspirin with one group left as the control. After 22 days, the rats were sacrificed and histologic sections of the fracture sites were examined in a blinded fashion for healing. The result was a dose-related response with increased doses relating to delayed fracture healing in the indomethacin group. There was a similar response in the aspirin group, though this was not statistically significant. Since then, there have been several other studies in animals that demonstrated delayed bone healing. Hogevold et al (3) took 72 rats and assessed the healing of intramedullary pinned osteotomies of the femur. He found that the group given indomethacin showed statistically significant decreases in bone strength, absorbed energy, and bending rigidity when mechanically tested in cantilever bending. Similarly, Altman et al (4) studied the effects of ibuprofen and indomethacin on rats with femur fractures. These results demonstrated evidence of delayed healing in both groups both by mechanical testing and histological evaluation. In addition, there are many other studies in animals that support such results. However, there are also studies in animals that have shown negative results, though these studies are fewer in number. More et al (5) examined 28 rabbits who were given tibia fractures. Six were given flunixim, an NSAID used in veterinary medicine, and six each were given low-dose and high-dose peroxicam. The remaining ten served as controls. After three weeks, there was no statistically significant difference in the healed fractures as measured by ankle stiffness or tibia torsional strength. Huo et al (6) examined 152 rats with femur fractures. Half were given ibuprofen and the other half were given nothing. Animals were sacrificed at intervals over twelve weeks, and the study found no significant differences between the treated and control groups with regards to biomechanical testing as well as histologic study. In contrast to studies using animal subjects, studies examining the effects of bone healing with NSAID therapy in humans are much fewer in number. Giannoudis et al (7) looked retrospectively at 377 patients treated with intramedullary nailing for femoral shaft fractures. Nonunion had occurred in 32 of them. Out of the remaining, the authors selected 67 patients similar in sex and age. They then examined differences, including postoperative NSAID use, between the two groups and found that a larger number of patients (62.5% vs. 13.4%) used NSAIDs in the nonunion group (p value = 0.000). Similarly, Butcher et al (8) retrospectively examined 94 patients who had sustained tibial fractures. He found that those who received NSAIDs as part of their treatment had an average length of time to union 7.6 weeks greater (p value = 0.0003) from 16.7 to 24.3 weeks. As for prospective studies in humans, there have only been two to date, and they have decidedly different conclusions. Burd et al (9) studied 112 patients who received ORIF of acetabular fractures and who also had concomitant long bone fractures. Those at risk for heterotopic ossification based on the surgical approach were randomized to receive either radiation or indomethacin; the remaining served as controls. The results demonstrated a higher incidence of nonunion of a long bone fracture in the indomethacin group (11 of 38 patients or 29%) versus in the non-indomethacin (radiation and control) group (5 of 74 patients or 7%). Adolphson et al. (10) conducted a randomized, double-blind, placebo-controlled trial examining 42 postmenopausal women with displaced Colles’ fracture. After closed reduction, patients were randomized to receive either piroxicam or placebo. Those who had received piroxicam were found to have no difference in fracture healing as measured by clinical function; they did, however, note significantly less pain. For those who do believe that NSAIDs delay fracture healing, even the mechanism is uncertain. NSAIDs are thought to either act locally at the fracture site or through hormonal effects. Zhang et al (11) reviewed the literature on cyclooxygenases and their role in bone repair. In his review, he states that the enzymes are implicated in key steps in bone repair including, chondrogenesis, chondrocyte differentiation, and osteoblast differentiation. In contrast, Edwall et al (12) demonstrated in rats that insulinlike growth factor I (IGF-I) was increased at 8 days after fracture and that this level was depressed by indomethacin administration, implicating both a role of IGF-I in fracture healing and indomethacin’s inhibitory response. In conclusion, there is theoretical reason to believe that NSAIDs may retard fracture healing, and animal studies have largely borne this out. Human studies, on the other hand do not wholly support this conclusion. While retrospective studies have mostly confirmed the results of animal studies, only two prospective studies have been conducted, and they reached opposing conclusions. While it may be likely that NSAIDs do, in fact, retard fracture healing, more research in humans needs to be done, especially if we are to deny patients the analgesic effects of NSAIDs. REFERENCES: 1. Rodeo SA, Maher SA, Hidaka C. What’s new in orthopaedic research. J Bone Joint Surg Am. 2004 Sep:86-A(9):2085-95. 2. Allen HL, Wase A, Bear WT. Indomethacin and aspirin: effect of nonsteroidal anti-inflammatory agents on the rate of fracture repair in the rat. Acta Orthop Scand. 1980 Aug;51(4):595-600. 3. Hogevold HE, Grogaard B, Reikeras O. Effects of short-term treatment with corticosteroids and indomethacin on bone healing: a mechanical study of osteotomies in rats. Acta Orthop Scand. 1992 Dec;63(6):607-11. 4. Altman RD, Latta LL, Keer R, Renfree K, Hornicek FJ, Banovac K. Effect of nonsteroidal antiinflammatory drugs on fracture healing: a laboratory study in rats. J Orthop Trauma. 1995;9(5):392-400. 5. More RC, Kody MH, Kabo JM, Dorey FJ, Meals RA. The effects of two nonsteroidal anti-inflammatory drugs on limb swelling, joint stiffness, and bone torsional strength following fracture in a rabbit model. Clin Orthop. 1989 Oct;(247):306-312. 6. Huo MH, Troiano NW, Pelker RR, Gundberg CM, Friedlaender GE. The influence of ibuprofen on fracture repair: biomechanical, histologic, and histomorphometric parameters in rats. J Orthop Res. 1991 May;9(3):383-90. 7. Giannoudis PV, MacDonald DA, Matthews SJ, Smith RM, Furlong AJ, De Boer P. Nonunion of the femoral diaphysis. The influence of reaming and non-steroidal anti-inflammatory drugs. J Bone Joint Surg Br. 2000 Jul;82(5):655-8. 8. Butcher CK, Marsh DR. Non steroidal anti-inflammatory drugs delay tibial fracture union. Abstract. Injury. 1996;(27):375. 9. Burd TA, Hughes MS, Anglen JO. Heterotopic ossification prophylaxis with indomethacin increases the risk of long-bone nonunion. J Bone Joint Surg Br. 2003 Jul;85(5):700-5. 10. Adolphson P, Abbaszadegan H, Jonsson U, Dalen N, Sjoberg HE, Kalen S. No effects of piroxicam on osteopenia and recovery after Colles’ fracture: a randomized, double-blind, placebo-controlled, prospective trial. Arch Orthop Trauma Surg. 1993;112(3):127-30. 11. Zhang X, Zyang Y, Schwarz EM, O’Keefe RJ. Cyclooxygenases and bone repair. Curr Opinion Orthop. 2001;(12):397-402. 12. Edwall D, Prisell PT, Levinovitz A, Jennishe E, Norstedt G. Expression of insulin-like growth factor I messenger ribonucleic acid in regenerating bone after fracture: influence of indomethacin. J Bone Miner Res. 1992 Feb;7(2):207-13.