CHEMISTRY 12BLR * ORGANIC CHEMISTRY
advertisement

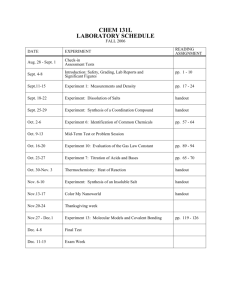
ORGANIC CHEMISTRY I CHEMISTRY 12AL LABORATORY SYLLABUS Fall 2012 Instructor: Bobbie Grey Phone: (951) 222-8270 e-mail: bobbie.grey@rcc.edu Office Location: MTSC 427 Office Hours: MW: 11:00 a.m. – 12:40 p.m. T: 11:00 a.m. – 2:00 p.m. Course Schedule: MW 2:20 p.m. – 5:30 p.m. Room: MTSC 405 *OR* MW 6:00 p.m. – 9:10 p.m. Room: MTSC 405 Lab Text: Mandatory: “Organic Chemistry Lab- Chemistry 12B” Pavia, Landgrebe, Bell, Taber, Clark. Brooks/Cole/Cengage Optional: “A Short Guide to Writing About Chemistry” Davis, Pechenik, Tyson. Pearson Important Laboratory Information (1) It is not possible to get a passing grade for the course without completing the laboratory. (2) Cheating will not be tolerated. Students caught cheating will receive a zero grade for the experiment and will be subject to dismissal from the class with a failing grade. Cheating includes (but is not limited to) turning in a report without doing the experiment, copying any portion of another student’s work and turning it in as your own, letting another student copy your work, interfering with another student's work, removing chemicals or glassware from the laboratory, or providing test questions or answers to other sections. (3) Attendance at your own laboratory meetings is mandatory. If you miss a laboratory you may not be able to make-up that laboratory. For one absence only, with a verifiable medical excuse, your laboratory score will be pro-rated. (4) Lab Preparation Write-ups (Pre-Labs) are to be completed before lab and are due at the beginning of the lab period in which the experiment is to be done. (5) Laboratory Reports are due 1 week following completion of the experiment, or as directed by the Instructor. Late reports will not be accepted. Chem 12AL - Fall Schedule 2012 Dates Aug. 27 & 28 Aug. 29 & 30 Sept. 4 Sept. 5 & 6 Exp # Handout Handout Sept. 10 & 11 Handout Sept. 12 & 13 Sept. 17 & 18 1A&C 2 A-D Sept. 19 & 20 3A&D Sept. 24 & 25 Sept. 26 & 27 4D Handout Oct. 1 & 2 5 Oct. 3 & 4 Oct. 8 & 9 Oct. 10 & 11 Oct. 15 & 16 Oct. 17 & 18 Oct. 22 & 23 Oct 24 & 25 5 6 6 12 A 11 11 12B 9C Oct. 29 & 30 Handout Oct. 31 & Nov. 1 8 Nov. 5 & 6 Nov. 7 & 8 Nov. 13 Handout Handout Nov. 14 & 15 7 Nov. 19 & 20 Nov. 21 Nov. 26 & 27 Nov. 28 & 29 Dec. 3 & 4 Dec. 5& 6 7 13 Title Safety Training & Lab Orientation, Using the Library for Chemistry 12A & 12B TBA Computer Modeling Molecular Models (bring model kits to lab!) Check-In Identification of an Unknown Solid by M.P. Solubility of Organic Compounds Crystallization (1/3 scale) Solubility of sulfaniliamide: 210mg/mL at 78°C, 14mg/mL at 0°C Extraction Technique (1/2 scale) Isolation of Aspirin Isolation of Caffeine from Tea (12A procedure) Continued Isolation of Eugenol from Cloves (1/2 scale) Continued Bromination of E-Stilbene Addition of Dichlorocarbene to Cyclohexene Continued Synthesis of Diphenylacetylene Monitoring a Reaction with Thin Layer Chromatography Carbocation Rearrangements: Benzopinacolone Start Chiral Crystal Lab Formation of Chiral Crystals Stereoisomers TBA Isolation of Chlorophyll and Carotenoid Pigments from Spinach Continued TBA Synthesis of t-Pentyl Chloride (Microscale) Organic Molecule Presentations Lab Exam Review & Check-Out Lab Exam FORMAT FOR LABORATORY NOTEBOOKS General a. b. c. d. e. I. The laboratory notebook may not be loose leaf or spiral bound (see (d) below). It must be designed so that it permanently contains the original pages of your Pre-lab (Part I), and Data and Observations (Part II). Use pen only. Textbooks, lab text, loose sheets of paper, etc. will not be allowed in the lab during sessions. All you need to carry out the laboratory must be written in your laboratory notebook prior to carrying out the experiment. Copies of lab notebook pages will be required. Duplicate-paged notebooks with carbon paper can be purchased from the bookstore. These are designed such that copies of all the original pages can be removed for submission to your Instructor. The original pages must remain securely attached to the lab notebook and must not be removed. Your Instructor may periodically inspect your notebook. Lab Preparation Write-up (Pre-Lab) This is Part I of your lab report. It must be written in your laboratory notebook. A copy of the original pages of this write-up will be collected prior to beginning any experiment and it will be returned after your entire lab report has been collected and graded. It will consist of: a. b. c. d. e. f. g. h. i. Your name, lab section, and date (on each page). Experiment Number & Title. Purpose: Concisely and in your own words, state the purpose of the experiment. You’ll want to use the names of your reactants and products when applicable and identify the type/name of reaction used. Scheme of reaction: Draw out the reaction scheme. This is not the detailed mechanism. That will come later. Table of reagents and products: Name, molecular formula, mass (or volume) used in experiment, molar mass, density (if applicable), boiling point, melting point, refractive index, and safety concerns. Equipment list Equipment set-up: sketch your complex reaction apparatus and label each part. You do NOT need to draw each piece of equipment in your equipment list. Outline of procedure: It is most worthwhile to show this in outline form; note precautions, including comments on safety in the margin. Answers to pre-lab questions. The copy of Part I must be completed and ready to turn in before you can begin the experiment. II. Data and Observations This section should be started on a new page of the notebook following Part I. A copy of Part II will be turned in at the end of the experiment as directed by your Instructor. This section should be completed during the laboratory session while doing the experiment and includes: a. b. c. Name, lab section, and date on each page. Experiment Number and Title Data and Observations: Weights, volumes, mp's and other observations or comments actually recorded during the lab session. All numbers should have labels attached. Clearly display data and observations of any final products. Note changes to procedure. III. Formal Lab Report This section is to be typed and done as homework after you have completed the experiment and includes: a. b. c. b. c. d. e. Title Name, lab section, etc. on each page. Introduction. Big picture -> Small picture -> Reason for experiment Scheme or mechanism of reaction (drawn in Chem-Draw when applicable). Summary of Results (in a table whenever possible): calculate theoretical and percent yields, melting point percent error etc. Also, include charts, graphs, diagrams, spectra, and pictures. Label each as a “Figure.” Discussion and Conclusions: In 5-10 sentences, comment on your results. If you put information in a figure, you MUST discuss it here. Do you think you got the product you were looking for? How do you know? What tests did you do? What did those tests tell you? Include an error analysis, describing problems that may have occurred, possible solutions, etc. What was learned? Post-lab questions (use Chem-Draw if chemical structures are needed). Turn in 1 week after completion of the experiment or as directed by your Instructor. Also, if applicable, turn in products in a properly labeled vial at this time. Parts I-III will be returned to you after grading. You should keep a copy of Part III. Lab reports will always be done individually even when a lab is done with a partner.