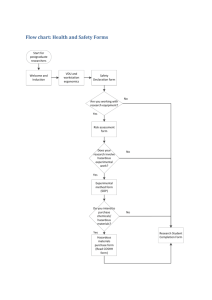
SOP 28 NGM SElect - Health and Safety
advertisement

2015/2016 FACULTY OF COMPUTING, ENGINEERING & SCIENCES – SCHOOL OF SCIENCES RISK ASSESSMENT FORM Procedure: Academics complete Risk Assessment for all practical classes/activities, Technical team for all support aspects and forward to Faculty Health & Safety Advisor, this is then reviewed on an annual basis Experimenters complete Risk Assessment in consultation with project advisor and technical staff as appropriate No laboratory work is to commence without a suitable and sufficient risk assessment being signed off by the Faculty Health & Safety advisor or for experimenters by the project advisor and Faculty Health & Safety Advisor/nominated individual Experimenters to keep copies of Risk Assessments when working in the laboratories Notes: The risk assessment must be reviewed when any changes are made to the equipment, materials, procedure or personnel Technical staff can stop experimental work if no risk assessment is in place, or if, in their opinion, there is a risk to safety □ Biology □ Forensics & Crime Science □ Geography Student: Name & email address: Ref No: SOP 27 Project advisor: STAFF: Mishele Barrigas Ethical consideration: N/A Date submitted/passed Title of project/module (include module number): General Standard of Practice/ Research Purposes ONLY Description of experimental procedure/practical session: Amplification and Processing of Human DNA Samples for Genetic Analysis using the NGM SElect™ PCR Amplification Kit (Life Technologies). A mix containing NGM primer set and NGM Mastermix to be prepared for all samples to be amplified, 7.5 µl distributed into PCR tubes along with 5 µl of DNA, Loaded into a thermal cycler and run using the set cycling parameters. Post amplification - samples are processed using a mixture of Hidi formamide and LIZ size standard mix followed by a 3 minute incubation at 95oC and a 3 minute cold treatment (on ICE). Samples are then transferred to a 3500 96-well plate and plate briefly centrifuged before either storing at -20oC or loaded onto the 3500 genetic analyser. Hazards inherent in the work, record details and possibility of risk/harm: (Equipment, procedures, invertebrate work, body fluid sampling etc.) Record precautions which will be taken: (e.g. Any standard operating procedures to follow, SAF codes, faculty policies) Electrical Hazards: Microfuge, vortex, thermal cycler, heating block. All equipment will be checked prior to use to ensure electrical testing has taken place and that no loose or exposed wires are visible. Possible personal harm from reagents. Hidi Formamide is toxic All reagents are used in very small volumes. Caution when handling Hidi Formamide - gloves always worn and small volumes used. All other reagents used in these processes are listed as “Non-hazardous”. All processes, and their hazards, leading up to this procedure. See SOP 11, SOP 14. For the preparation and running of the 3500, and associated hazards – SPECIALLY TRAINED STAFF ONLY. See SOP 9. COSSH assessment (harmful substances) Minimum handling precautions Hazardous to the Aquatic Environment (W) Acute Toxicity (T+) Gases Under Pressure (G) Fume cupboard (F) Safety glasses (SG) Microbiological cabinet (Cab) Laminar flow cabinets (LF) Gloves (GL) Face mask (M) Respirator (R) Other All INFORMATION CAN BE FOUND WITHIN MSDS (MATERIAL SAFETY DATA SHEETS) ON THE INTERNET, SCIENCES CHEMICAL DATABASE ON-LINE OR WITHIN EACH OF THE LABORATORIES Corrosive (C) Caution (H, I) Explosive (E) Flammable (F) Longer Term Health Hazards (M) Oxidising (O) Chemicals involved (including Products): COSHH information as above: Minimum handling precautions: NGM SElect Mastermix - Gl Quantity to be used: ml/g/% solution/M PER REACTION 5 µl 2 NGM Select Primer set Allelic ladder LIZ size standard PCR Water Microorganisms: Classification: Gl 2.5 µl Gl 1 µl Gl 0.5 µl Gl 5 µl Minimum handling precautions: Other Materials: Hazards: Minimum handling precautions: 007 control DNA Sample DNA - Gl Gl Quantity to be used: PER REACTION - 5 µl 5 µl Do any of the above substances have a workplace exposure limit (WEL) please state value and precautions: No Disposal information (How will all reactants/products be disposed of?) All waste volumes are minimal and can to be disposed of, contained within either 0.2ml, 1.5mlor 96 well plates, in the waste hazard bags and autoclaved before disposal. Hidi formamide waste, however small, is to be stored as hazardous waste until collection by a chemical disposal company. Have you checked all materials used are not hazardous to the environment? YES Any special conditions specified as part of the permission to carry out the work/procedure and actions needed to minimise risk e.g. adherence to HTA or body fluid policy 1.52, completion of fieldwork risk assessment etc. Project advisor/Academic comments: (Any disability issues to be aware of?) Staff/Project advisor- What level of risk do you assign with this work? High Medium Low Date: 16 November. 2015 Faculty H&S approval: Audra Jones Date: 17.11.2015 Date of Review 3 4