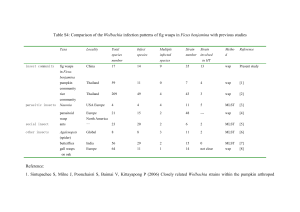
The prevalence of Wolbachia infection in common pest insects of
advertisement

Summer Envisionship Initial Report Matthew Allen and Tony DeRosa Project Title: Determining the prevalence of Wolbachia infection and to identify the Wolbachia strains present in common pest insects of organic and GMO-free crops in western Wisconsin. Project Introduction: We are looking at insect pests of organic and no-GMO crops in western Wisconsin. Organic and non-GMO farming is increasing in its popularity in western Wisconsin and throughout the country. For these types of farmers, insect pest management is very different from that of other commercial farmers. Non-organic farmers generally use chemical pesticides and/or GMO crops to manage insect pests. Organic and non-GMO farmers must rely on manual and natural control of insect pests. We were interested in determining the Wolbachia infection rate and if possible the strains involved in the infection for these insect pests. Potentially, an understanding of the Wolbachia infection rates and knowledge of the strains involved in the Wolbachia infection could lead to novel non-chemical pest control. That portion is outside the scope of this project but it was a motivator for our interest in finding out about the presence of Wolbachia in these insect groups. Sample Sites: We have collected samples from 5 different organic farms in northwestern Wisconsin. We sampled farms from Eau Claire, Durand, Glenwood, Augusta, and Stanley. The farms were in Eau Claire, Chippewa, Dunn, and Pepin counties. Methods and Procedures: For collections, we collected insects in the field using nets and tweezers. All insects were placed live into 95% ethanol on ice and then stored in the freezer. When possible, extractions and amplifications were done on the same day as the collection. Amplified samples were kept refrigerated until run on the gels and then were frozen. For extraction and amplification, Sigma Extract-N-AmpTM Tissue PCR Kits (product number XNAT2-1KT) were used. Extraction Protocol: 1. In a 1.5 ml microtube, combine 100µl of Extraction Solution and 25µl of Tissue Prep Solution and mix by pipetting up and down 2. Add 2-10mg piece of insect abdomen or if small, whole insect to the solution and macerate using a microtube pestle 3. Incubate at room temperature for 10 minutes 4. Incubate at 95°C for 3 minutes 5. All 100µl of Neutralization Solution B and mix by vortexing 6. Pipet off the liquid into a new tube and store at 4°C or continue to PCR immediately Amplification Protocol: 1. In a PCR tube, combine 7µl PCR quality water, 10µl Extract-N-Amp PCR Ready Mix, 2µ wspec F primer, 2µl wspec R primer, and 4µl of DNA for a total volume of 25µl 2. Run in thermalcycler with the following settings a. 95°C 2 min 1 cycle b. 94°C 30 sec 38 cycles 55°C 45 sec 72°C 90 sec c. 72°C 10 min 1 cycle d. 4°C indefinitely Electrophoresis Protocol: 1. 2. 3. 4. Prepare a gel at 1% agarose using TBE Mix 10µl of sample with 2µl of FOTO/Vision stain on Parafilm Put 10µl of sample and stain mixture into the gel Run at 100V for 30 minutes Preliminary Results: We collected and tested samples from 5 different farms. Farm 1 was in Eau Claire County. Farm 2 was in Dunn County. Farm 3 was in Pepin County. Farm 4 was in Eau Claire County. Farm 5 was in Chippewa County. The following insects were collected at each site and in parentheses are the number of positive results and the number of individuals tested. Farm 1: Colorado Potato Beetle – Coleoptera Flea Beetle – Coleoptera Squash Bug – Hemiptera s.o. Heteroptera Cabbage Butterfly – Lepidoptera (0/4) (13/13) (8/15) (0/2) Farm 2: Colorado Potato Beetle – Coleoptera Cabbage Butterfly (larvae) – Lepidoptera (0/5) (0/5) Farm 3: Cucumber Beetle – Coleoptera Fruit Fly – Diptera (0/5) (3/15) Farm 4: Flea Beetle – Coleoptera (12/15) Farm 5: Colorado Potato Beetle (immature) – Coleoptera Cabbage Butterfly – Lepidoptera Cucumber Beetle – Coleoptera (0/5) (0/5) (0/4) See attached spread sheet for details of each sample run and gel photos. Collections tab gives details on the collection dates and times. Data tab gives all of the data and explanations of codes. Individual gel tabs shows data for each gel and the gel photo