Experiment #15-3 Name Endothermic & Exothermic Reactions
advertisement

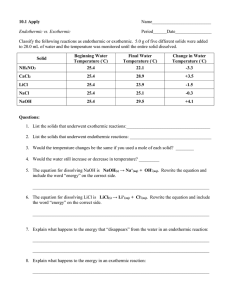
Experiment #15-3 Endothermic & Exothermic Reactions Name Group # Per.# PURPOSE: In this activity you will test four reactions to determine if they are endothermic or exothermic, determine the heat of reaction in kJ/mol of solid , and determine if the reactions are physical changes or chemical changes. PROCEDURE: 1. Use approximately the following amounts of solids and liquids in your measurements. Reaction Solid Liquid A Ammonium chloride (6-7 grams) Distilled water (30 mL) B Calcium chloride (9-10 grams) Distilled water (30 mL) C Magnesium metal (2 cm) Hydrochloric acid 0.5 M (30 mL) D Sodium hydrogen carbonate (10 grams) Acetic acid 1.0 M (30 mL) 1. Use a graduated cylinder to determine the mass of the liquid. (Assume all liquids have a density of 1.0g/ml. Add the liquid to the calorimeter. 2. Record initial temperature of the liquid. 3. With constant stirring, add the solid to the calorimeter and determine the max or min temp. DATA: Reaction Mass of Solid: A 6.61 Mass of Liquid: 30. g TInitial (+/- 0.1℃) TFinal (+/- 0.1℃) Observations 22.7 9.9 B 9.16 30. g 22.6 43.7 C 3.79 30. g 23.3 28.5 The solid dissolved The solid dissolved Gas was evolved D 10.0 30. g 23.2 16.6 Gas was evolved Write balanced chemical equations for all four reactions. Remember to include heat on the reactant or product side, as appropriate. A B C D In each case, circle the appropriate answer. Complete the following table to indicate whether each reaction represents a physical or chemical change and whether it is exothermic or endothermic. Reaction P or C Change? Endothermic or Exothermic Evidence for chemical or physical changes A B C D Determine the energy for each reaction. (Assume the specific heat all liquids equals 4.18 J/g℃.) Reaction (A) Energy per mole of ammonium chloride added: Determine the Joules of energy gained/lost by the solution. Determine the J/mole of solid added. Reaction (B) Energy per mole of calcium chloride added: Determine the Joules of energy gained/lost by the solution. Determine the J/mole of solid reacted. Reaction (C) Energy per mole of magnesium added: Determine the Joules of energy gained/lost by the solution. Determine the J/mole of solid reacted. Reaction (D) Energy per mole of sodium hydrogen carbonate added: Determine the Joules of energy gained/lost by the solution. Determine the J/mole of solid reacted.