10.1 Apply Name__________________________ Period______Date________________
advertisement
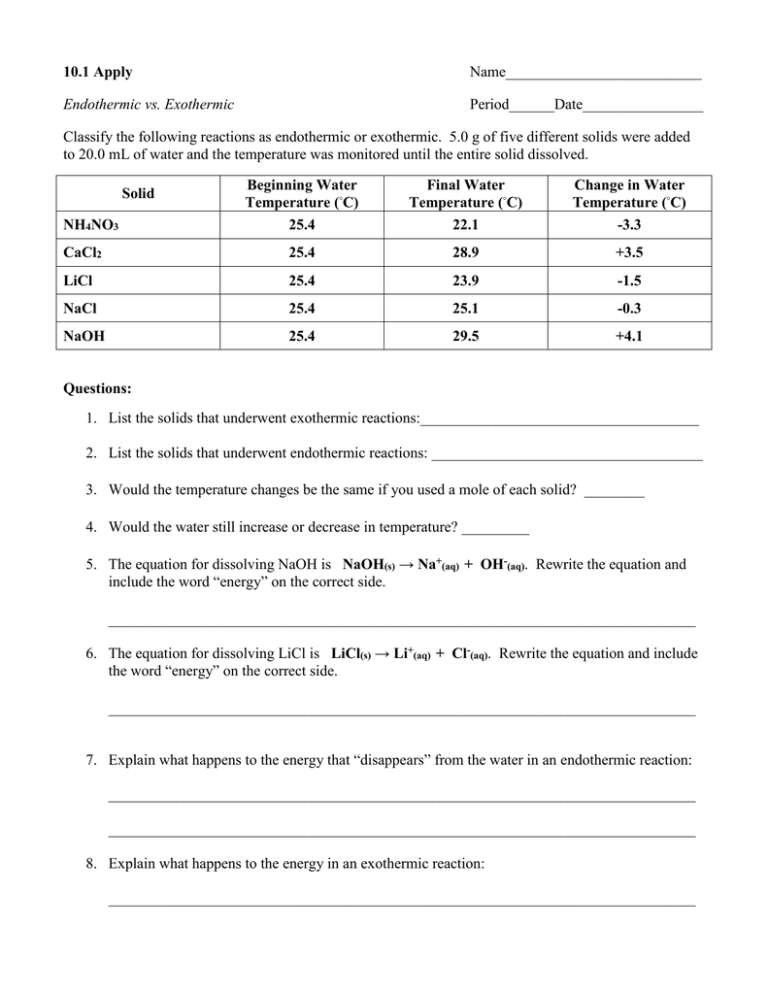
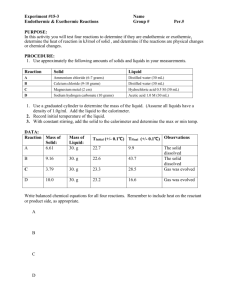
10.1 Apply Name__________________________ Endothermic vs. Exothermic Period______Date________________ Classify the following reactions as endothermic or exothermic. 5.0 g of five different solids were added to 20.0 mL of water and the temperature was monitored until the entire solid dissolved. Beginning Water Temperature (◦C) Final Water Temperature (◦C) Change in Water Temperature (◦C) NH4NO3 25.4 22.1 -3.3 CaCl2 25.4 28.9 +3.5 LiCl 25.4 23.9 -1.5 NaCl 25.4 25.1 -0.3 NaOH 25.4 29.5 +4.1 Solid Questions: 1. List the solids that underwent exothermic reactions:_____________________________________ 2. List the solids that underwent endothermic reactions: ____________________________________ 3. Would the temperature changes be the same if you used a mole of each solid? ________ 4. Would the water still increase or decrease in temperature? _________ 5. The equation for dissolving NaOH is NaOH(s) → Na+(aq) + OH-(aq). Rewrite the equation and include the word “energy” on the correct side. ______________________________________________________________________________ 6. The equation for dissolving LiCl is LiCl(s) → Li+(aq) + Cl-(aq). Rewrite the equation and include the word “energy” on the correct side. ______________________________________________________________________________ 7. Explain what happens to the energy that “disappears” from the water in an endothermic reaction: ______________________________________________________________________________ ______________________________________________________________________________ 8. Explain what happens to the energy in an exothermic reaction: ______________________________________________________________________________ 10.2 Apply – Which is better, Natural Gas or Propane? Many homes heat and cook with either methane (natural gas, CH4) or propane (C3H8). Here are the equations for their combustion including the standard enthalpy changes. CH4 + 2 O2 C3H8 + 5 O2 CO2 + 2 H2 O ∆H◦ = -890 kJ → 3 CO2 + 4 H2 O ∆H◦ = -2043 kJ → Questions: 1. Calculate the amount of heat released when 5.50 g of methane is burned. 2. Calculate the amount of heat released when 5.50 g of propane is burned. 3. Which product gives off more heat per gram? _______________ 4. What is the amount of heat given off when 1 mole of methane is burned? ________________ 5. What is the amount of heat given off when 1 mole of propane is burned? ________________ 6. Which fuel gives off more heat per mole? _______________ 7. Heating fuel is typically rated in terms of British Thermal Units (BTUs) instead of kilojoules. Given: 1 kJ = 0.948 BTU, calculate the heat released during combustion of one mole of methane and one mole of propane in BTUs. 8. Heating fuel is typically sold by mass. Based on this, which gas is the more economical heating fuel? _________________ Explain! _____________________________________________________________________________ _____________________________________________________________________________