Chemistry Study Guide: Matter, Reactions, and Nuclear Chemistry
advertisement
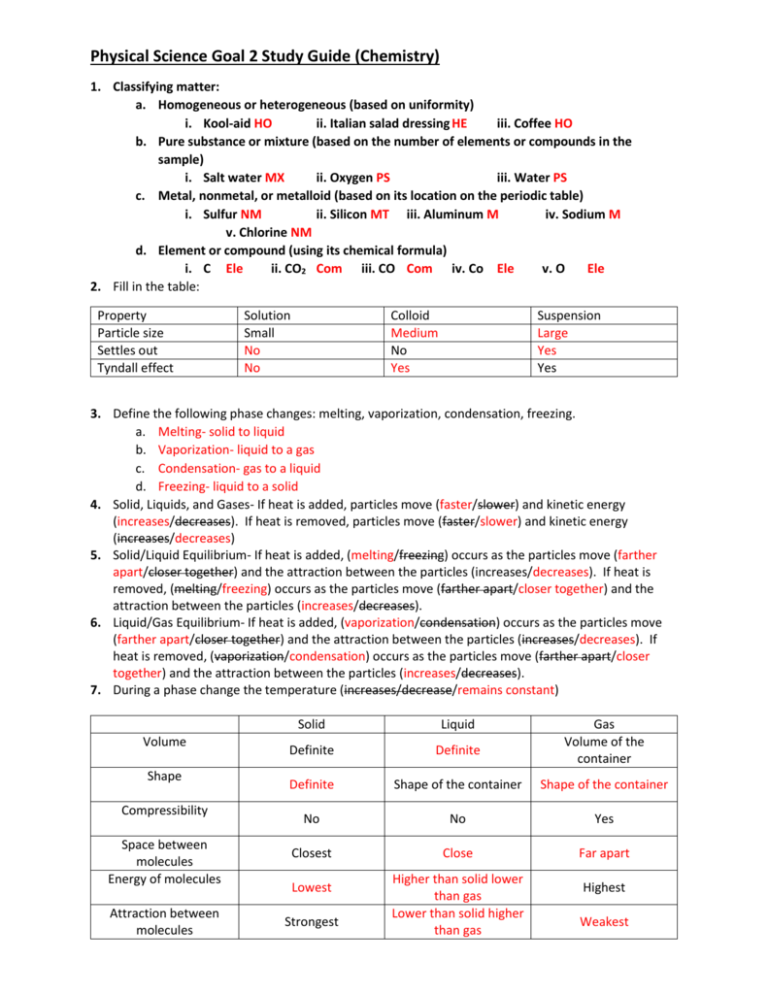
Physical Science Goal 2 Study Guide (Chemistry) 1. Classifying matter: a. Homogeneous or heterogeneous (based on uniformity) i. Kool-aid HO ii. Italian salad dressing HE iii. Coffee HO b. Pure substance or mixture (based on the number of elements or compounds in the sample) i. Salt water MX ii. Oxygen PS iii. Water PS c. Metal, nonmetal, or metalloid (based on its location on the periodic table) i. Sulfur NM ii. Silicon MT iii. Aluminum M iv. Sodium M v. Chlorine NM d. Element or compound (using its chemical formula) i. C Ele ii. CO2 Com iii. CO Com iv. Co Ele v. O Ele 2. Fill in the table: Property Particle size Settles out Tyndall effect Solution Small No No Colloid Medium No Yes Suspension Large Yes Yes 3. Define the following phase changes: melting, vaporization, condensation, freezing. a. Melting- solid to liquid b. Vaporization- liquid to a gas c. Condensation- gas to a liquid d. Freezing- liquid to a solid 4. Solid, Liquids, and Gases- If heat is added, particles move (faster/slower) and kinetic energy (increases/decreases). If heat is removed, particles move (faster/slower) and kinetic energy (increases/decreases) 5. Solid/Liquid Equilibrium- If heat is added, (melting/freezing) occurs as the particles move (farther apart/closer together) and the attraction between the particles (increases/decreases). If heat is removed, (melting/freezing) occurs as the particles move (farther apart/closer together) and the attraction between the particles (increases/decreases). 6. Liquid/Gas Equilibrium- If heat is added, (vaporization/condensation) occurs as the particles move (farther apart/closer together) and the attraction between the particles (increases/decreases). If heat is removed, (vaporization/condensation) occurs as the particles move (farther apart/closer together) and the attraction between the particles (increases/decreases). 7. During a phase change the temperature (increases/decrease/remains constant) Volume Shape Compressibility Space between molecules Energy of molecules Attraction between molecules Solid Liquid Definite Definite Definite Shape of the container Shape of the container No No Yes Closest Close Far apart Lowest Strongest Higher than solid lower than gas Lower than solid higher than gas Gas Volume of the container Highest Weakest 8. How are evaporation and vaporization different? a. Evaporation occurs below the boiling point. b. Vaporization occurs at the boiling point. 9. Solutions: a. Solute- the substance that is dissolved in the solvent. b. Solvent- the substance that dissolves the solute. c. “Likes dissolve Likes” i. (Polar/Nonpolar) solvents dissolve polar and ionic solutes. ii. (Polar/Nonpolar) solvents dissolve nonpolar solutes. iii. Nonpolar solutes are (soluble/insoluble) in polar solvents. 10. Practice: Solubility Curve a. What is the solubility of KClat 5C? 28 g b. What is the solubility of KClat 25C? 35 g c. What is the solubility of Ce2(SO4)3at 10C? 15 g d. What is the solubility of Ce2(SO4)3 at 50C? 5 g e. At 90C, you dissolved 10 g of KCl in 100. g of water. Is this solution saturated or unsaturated? How do you know? Unsaturated; below the line about 53 g of KCl can be dissolved at 90 degrees C. f. A mass of 100 g of NaNO3 is dissolved in 100 g of water at 80ºC. Is the solution saturated or unsaturated? unsaturated g. As the solution is cooled, at what temperature should solid first appear in the solution? Explain. 35 degrees C. The solubility of NaNO3 at 35 degree C is 100 g after that point excess solute will settle out of solution. h. Which compound is most soluble at 20 ºC? KI i. Which is the least soluble at 40 ºC? Ce2(SO4)3 j. Which substance on the graph is least soluble at 10C? KClO3 11. Practice: Density Problems a. 100 grams of a liquid completely fill a 200 mL bottle. What is the density of the liquid? 0.5 g/mL b. A solution has a density of 1.50 g/mL. How many grams are needed to obtain 10.0 mL of solution? 15 g c. If a block of copper measures 2.00 cm x 4.00 cm x 5.00 cm and weighs 356 grams, what is its density? 8.9 g/cm3 d. The density of mercury is 13.6 g/mL. i. What is the mass of 8.20 mL of mercury? 111.5 g ii. What volume would 120 grams of mercury occupy? 8.82 mL 12. Properties of Matter: Property Melting Point Boiling Point Density Appearance Conductivity Metals High High High Shiny Yes Nonmetals Low Low Low Dull No Brittle or Malleable Malleable and ductile brittle 13. Fill in the table: Protons +1 1 amu Nucleus Neutrons 0 1 amu Nucleus Electrons -1 1/2000 amu Electron cloud Charge Relative Mass (amu) Location 14. Atomic math: a. protons = atomic number b. electrons = # protons – charge c. neutrons = mass number - # protons d. mass number = # neutrons + # protons e. Atomic number = number found on the periodic table (or # protons) f. Charge = # of protons - # electrons 15. Use isotopic notation to write symbols for various isotopes. 𝟑𝟕 𝟑𝟓 1Chlorine-35 𝟏𝟕𝑪𝒍 𝟏𝟕𝑪𝒍 Mass Number 35 37 35 Atomic Number 17 17 17 Protons 17 17 17 Neutrons 18 20 18 Electrons 17 17 18 16. Draw Bohr models from hydrogen to argon including common isotopes and ions. (in class) a. Carbon-14 atom b. Chloride ion (-1) c. Sodium atom 17. Construct dot diagrams, a shorthand notation for Bohr models, using the element symbol and dots to represent electrons in the outermost energy level. (in class) a. Sulfur b. Lithium c. Aluminum 18. Fill in the following table: 1A Valence 1 electrons Oxidation +1 number Gain/lose Lose 1 electons 2A 2 3A 3 4A 4 5A 5 6A 6 7A 7 8A 8 +2 +3 +/- 4 -3 -2 -1 0 Lose 2 Lose 3 Lose/gain Gain 3 4 Gain 2 Gain 1 Already stable 19. As you go down a group of metals the reactivity increases, and as you go down a group of nonmetals the reactivity decreases. 20. Fill in the table What happens to the electrons? What types of elements are involved? Ionic Transferred Covalent Shared Metallic Sea of electrons Metal and a nonmetal Two nonmetals two metals 21. Name and write formulas for simple binary compounds containing a metal and nonmetal using representative elements and compounds involving common polyatomic ions: ammonium, acetate, nitrate, hydroxide, carbonate, sulfate, phosphate. Chemical Name Chemical Formula Chemical Formula Chemical Name Potassium oxide K2O Na2S Sodium sulfide Lithium nitride Li3N Na3P Sodium phosphide Beryllium sulfide BeS MgBr2 Magnesium bromide Barium chloride BaCl2 CaO Calcium oxide Beryllium fluoride BeF2 MgS Magnesium sulfide Lead(IV) sulfide PbS2 SnCl2 Tin(II) chloride Potassium nitrate KNO3 CaCO3 Calcium carbonate Ammonium fluoride NH4F Ba(OH)2 Barium hydroxide Aluminum phosphate AlPO4 MgSO4 Magnesium sulfate Potassium acetate KC2H3O2 K2CO3 Potassium carbonate 22. Name and write formulas for binary compounds of two nonmetals using Greek prefixes. Chemical Name Chemical Formula Chemical Formula Chemical Name Carbon monoxide CO CO2 Carbon dioxide Sulfur dioxide SO2 N2O Dinitrogen monoxide Carbon tetrachloride CCl4 N2O5 Dinitrogen pentoxide Sulfur trioxide SO3 PBr3 Phosphorus tribromide 23. Use coefficients to balance simple chemical equations involving elements and/or binary compounds. a. 2H2O 2H2 +1O2 b. 1ZnS + 2O2 1ZnSO4 c. 2Mg + 1O2 2MgO d. 2Na + 2H2O ___H2 + 2NaOH e. ___Li2CO3 + 2HF 2LiF + ___H2CO3 f. 2NH3 1N2 + 3H2 g. 2Al + 3H2SO4 ___Al2(SO4)3 +3H2 h. ___NaOH + ___HCl ___NaCl + ___H2O 24. Classify the above chemical reactions in question 27 as one of the four types. a. Decomposition b. Synthesis c. Synthesis d. Single replacement e. Double replacement f. Decomposition g. Single replacement h. Double replacement 25. Fill in the table: Identifying ion (H+ or OH-) pH range Taste Feel Litmus paper test Phenolphthalein test Reacts with metals Reacts with carbonates Reacts with fats/oils Conductivity Acids H+ 0-7 Sour Sticky Red Clear, colorless Produces hydrogen gas Yes (produces carbon dioxide) No Yes Bases OH7-14 Bitter Slippery Blue Pink No No Produces soap Yes 26. When an acid reacts with a base, the two products will be a salt and water. This is called a neutralization reaction. 27. Fill in the table: Symbol Composition Mass Penetrability Change in the mass number Change in the atomic number Alpha 4 2𝐻𝑒 or α 2 protons 2 neutrons 4 amu Paper Decreases by 4 Beta 0 −1𝑒 or β 1 electron Gamma Γ Energy 1/2000 amu Wood No change no mass Lead or concrete No change Decreases by 2 Increases by 1 No change 28. Fill in the table: Splitting or joining Required conditions Energy released Nature of the products Fission Splitting of a very large nucleus Room temperature A lot of energy Radioactive Fusion Combining of two smaller nuclei Temp. conditions like the Sun A lot more energy not radioactive 29. Half-life : the amount of time it will take half of the radioactive particles to undergo decay. a. What is the half-life of a 100.0 g sample of nitrogen-16 that decays to 12.5 grams in 21.6 seconds? 7.2 seconds b. All isotopes of technetium are radioactive, but they have widely varying half-lives. If an 800.0 gram sample of technetium-99 decays to 100.0 g of technetium-99 in 639,000 years, what is its half-life? 213,000 years c. A 208 g sample of sodium-24 decays to 13.0 g of sodium-24 within 60.0 hours. What is the half-life of this radioactive isotope? 15 hours d. If the half-life of iodine-131 is 8.10 days, how long will it take a 50.00 g sample to decay to 6.25 g? 24.3 days e. The half-life of hafnium-156 is 0.025 seconds. How long will it take a 560 g sample to decay to one-fourth of its original mass? 0.050 seconds f. Chromium-48 has a short half-life of 21.6 hours. How long will it take 360.00 g of chromium-48 to decay to 11.25 g? 108 hours g. Potassium-42 has a half-life of 12.4 hours. How much of an 848 g sample of potassium-42 will be left after 62.0 hours? 26.5 g