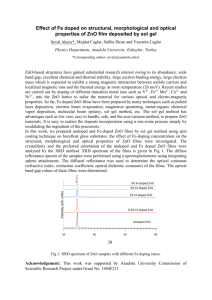
Kinetic decomposition of ozone, geosmin, and 2
advertisement

Kinetic decomposition of ozone, geosmin, and 2-methylisoborneol during catalytic ozonation Winn-Jung Huang,1,* Cheng-Feng Hsu2 and Yao-Ming Wang1 1 Department of Safety, Health and Environmental Engineering, Hungkuang University, Taichung 433, Taiwan 2 Department of Soil and Water Conservation, National Chung Hsing University, Taichung 402, Taiwan ABSTRACT Two taste and odor (T&O) causing compounds, geosmin (GSM) and 2methylisoborneol (2-MIB) were oxidized by zinc oxide (ZnO) catalyzed ozonation. The use of a 2.5 mg O3L-1 (52 μM) and 500 mg ZnO L-1 catalyst yields 96-97% GSM and 2-MIB conversion in 30 min; this experimental result compares favorably with the 53-58% obtained without a catalyst. The overall reaction rate was enhanced by the presence of ZnO; the most significant improvement in the overall rate constants (koverall) was observed at 500 mg ZnO L-1, whereby over four-fold increases in koverall value was observed. The experimental electron paramagnetic resonance (EPR) spectra confirmed that the formation of more hydroxyl radicals (•OH) in the ZnO catalytic ozonation process increases the T&O removal rate. Based on ozone decomposition kinetics and adsorption capacity of ZnO, the initial rate of disappearance of T&O correlates with the Langmuir-Hinshelwood kinetic model. The relationships between reaction rates and reactant concentrations were derived from the kinetic parameters determined experimentally for each reaction system. A comparison indicates that the kinetic rate constant for catalytic ozonation is 175-260% higher than that of the system of ozonation process due to increased production of hydroxyl radicals by an ozone combined with ZnO catalyst. KEYWORDS: Taste, odor, geosmin, 2-methylisoborneol, zinc oxide, catalytic ozonation, hydroxyl radical