Effect of solution molarity on the structural properties of ZnO
advertisement

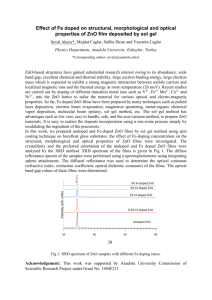
Effect of solution molarity on the structural properties of ZnO nanopowder synthesized by microwave-assisted hydrothermal synthesis Zinc oxide (ZnO) is an important multifunctional material with extensive applications in electronics, photoelectronics [1], sensors [2], and optical devices [3]. Especially for the application of gas sensors is preferred porous microstructure materials such as ZnO due to controlling pore size [4]. Additionally, ZnO is an impressive potential semiconductor with a direct wide band gap (3.37 eV), a large exciton binding energy of 60 meV, and excellent chemical and thermal stability [5]. To date, remarkable efforts have been dedicated to fabricate different ZnO nanostructures, including nanoflowers [6], nanorods [5], nanosheets [7] and so on, because of their strong size or morphology-dependent properties or device performances. ZnO nanopowder have been synthesized by many methods such as microwave-assisted hydrothermal synthesis [8], hydrothermal method [9] and vapor transport process [10]. Compared with conventional hydrothermal method, microwave hydrothermal synthesis has attracted wide interests due to its unique effects such as energy saving, higher reaction rates, rapid volumetric heating, low reaction temperature, homogeneous thermal transmission, higher selectivity and higher yields of products. In the present study, ZnO nanopowders were synthesized by microwave-assisted hydrothermal method at different molarities (0.1M-0.4M) of zinc acetate dihydrate. The effects of solution molarity on the structural and morphological properties of ZnO nanopowders were investigated by using X-ray diffraction (XRD) and field emission scanning electron microscopy (FE-SEM), respectively. XRD results showed that all the ZnO nanopowders are in crystalline form with hexagonal wurtzite phase and high purity. The crystallite size and texture coefficient values of ZnO nanopowders were calculated. The intensity of peaks of ZnO nanopowders gradually increased with the decreasing molarity. Also, a significant effect on the surface morphology was observed because of the variation of solution molarity. Acknowledgement This work was supported by Anadolu University Commission of Scientific Research Project under Grant no. 1306F243. [1] M. Law, L. E. Greene, J. C. Johnson, R. Saykally, P. Yang, Nature Mater. 4 (2005) 455–459. [2] J. X. Wang, X. W. Sun, Y. Yang, H. Huang, Y. C. Lee, O. K. Tan, L. Vayssieres, Nanotechnology. 17 (2006) 4995–4998 [3] J. Suehiro, N. Nakagawa,S. I. Hidaka, M. Ueda, K. Imasaka, M. Higashihata,T. Okada, M. Hara, Nanotechnology17(2006)2567–73 [4] S. Roy, S. Basu, Bull. Mater. Sci. 25 (2002) 513–515 [5] J.J. Wu, S.C. Liu, Adv. Mater. 14 (2002) 215–218 [6] J.P. Liu, X.T. Huang, Y.Y. Li, K.M. Sulieman, F.L. Sun, X. He, Scr. Mater. 55 (2006) 795. [7] C. Y. Lin, Y. H. Lai, H. W. Chen, J. G. Chen, C. W. Kung, R. Vittala, K. C. Ho, Energy Environ. Sci. 4 (2011) 3448 [8] A.P. De Moura, R.C. Lima, M.L. Moreira, D.P. Volanti, J.W.M. Espinosa, M.O. Orlandi, P.S. Pizani, J.A. Varela, E. Longo, Solid State Ionics. 181 (2010) 775–780 [9] Y. Wang, M. Li, Mater. Lett. 60 (2006) 266–269 [10] J. G. Lu, Z. Z. Ye, J. Y. Huang, L. P. Zhu, and B. H. Zhao, Z. L. Wang, S. Fujita, Appl. Phys. Lett. 88 (2006) 063110