300715103607Abstractsubmissiontemplate
advertisement

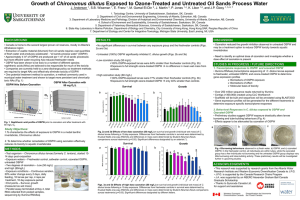
SusChemE 2015 International Conference on Sustainable Chemistry & Engineering October 8-9, 2015, Hotel Lalit, Mumbai Degradation of Reactive Black 5 Azo Dye Using Catalytic Ozonation with MgO Nikita P. Chokshi1, Bhargav Mehta2, J. P. Ruparelia3 1, 2, 3 Chemical Engineering Department, Institute of Technology, Nirma University nikita.chokshi@nirmauni.ac.in, 13mche05@nirmauni.ac.in, jr@nirmauni.ac.in Abstract: Water pollution is a one of the major problem in all over the world. One of the major source of water pollution is untreated sludge and that it accounts for the death of more than 14000 people daily. Water is a substance necessary for life, without water no one can imagine the life. Only few percentage of fresh water is available for living being out of which some percentage is in the form of ice. Sea water and sea ice constitutes of the 97% of the total remaining water. Due to scarcity of Water, it has become a necessary means to Re-Cycle and Re-Use water. [1-3] Micro pollutants in wastewater are a challenge to wastewater professionals. The presence of contaminants in WWTP effluents may cause a severe risk for the drinking water preparation. Upon discharge of the effluent into the receiving water body dilution and further degradation will occur. Nevertheless, some of the compounds might enter the drinking water treatment process, especially the process of those drinking water companies which produce drinking water from surface water. [4, 5]. Advanced Oxidation processes are widely used in treatment of wastewater because it generates •OH radical. This radical tend to be highly unstable and, therefore, highly reactive because one of their electrons is unpaired. Oxidation reactions that produce radicals tend to be followed by additional oxidation reactions between the radical oxidants and other reactants (both organic and inorganic) until thermodynamically stable oxidation products are formed. The ability of an oxidant to initiate chemical reactions is measured in terms of its oxidation potential. The most powerful oxidants are fluorine, hydroxyl radicals (•OH), ozone, and chlorine with oxidation potentials of 2.85, 2.70, 2.07 and 1.49 electron volts, respectively. The end products of complete oxidation (i.e., mineralization) of organic compounds are carbon dioxide (CO2) and water (H2O) [6, 7, 8]. Catalytic ozonation has gained highly increasing attention during past years, because of its good effectiveness in the degradation of organic pollutants. Organics can be oxidized by catalytic ozonation in the room temperature and pressure, which are hard to dissociate by single ozonation. Catalytic ozonation shows great advantages in refractory organics in water, and is expected to be the powerful and valuable technology in water treatment [9-11]. In this paper the catalytic ozonation of Reactive Black 5 (RB5) solution in the presence of MgO catalysts was investigated in a laboratory scale batch reactor. The effects of solution pH (3–12), reaction time, MgO dosage (0.05-1 g/L), and initial dye concentration (100–500 ppm) on color and TOC removal were evaluated, and the findings were compared to those of ozonation without a catalyst. The results indicate that addition of MgO crystals greatly accelerate the degradation of RB5 dye. Figure 1 Catalytic ozonation experiment set up 1. Oxygen cylinder 2. Ozonator 3. Reactor 4. Magnetic stirrer 5. KI solution References: [1] Guo, Y., Yang, L., Cheng, X., Wang, X., Environmental & Analytical Toxicology, 2012, 2-7. [2] Lucas, M.S., Mouta, M., Pirre, A., Peres, J.A., Water and Science Technology, IWA Publishing, 2009. [3] Eric, S.E., “Municipal Waste Water Treatment Plant (WWTP) Effluents”, RIWA, 2007, 5-8. [4] Sperling, M.V., “Wastewater characteristics, Treatment and Disposal”, IWA publishing, 2007, vol. 1, 6-11. [5] Abdel-Raouf, N., Al-Homaidan, A.A., Ibraheem, I.B.M., Saudi Journal of Biological Sciences, 2012, 19, 257-275 [6] Kommineni, S., Zoeckler, J., Stocking, A., Sun Liang., Flores, A., Kavanaugh M., Rodriguez, R., Browne, T., Roberts, R., Brown, A., Stocking, A., “Advanced Oxidation Processes”, Chap. 3, 111-113. [7] Advanced Oxidation Processes for Wastewater Treatment, International Journal of Photo-energy, Volume 2013 (Article ID 683682). [8] Grote, B., Skills Tech TAFE, Parklands, Gold Coast, 2012, 17-19. [9] Polat, D., Yucel, H., Ozbelge, T., Yilmazer, U., Catalytic Ozonation Of Industrial Textile Wastewaters In A Three Phase Fluidized Bed Reactor, Middle East Technical University, 2010, 4-12. [10] Hordern, B.K., Ziolek, M., Nawrocki, J., Applied Catalysis B: Environmental 46, 2003, 639–669. [11] Nawrocki, J., Applied Catalysis B: Environmental 142– 143, 2013, 465– 471.