Supplemental Material
advertisement

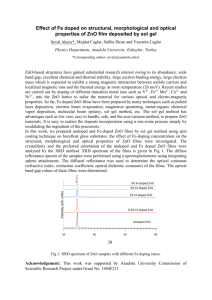
Supplemental Material Enhanced UV-violet electroluminescence and spectral narrowing from ZnO quantum dots/p-GaN heterojunctions by using high-k HfO2 electron blocking layer Xiaoming Mo,1) Hao Long,1) Haoning Wang,1) Songzhan Li,1,3) Zhao Chen,1) Jiawei Wan,1) Yamin Feng,4) Yuping Liu,1) Yifang Ouyang,2) and Guojia Fang1,a) 1 School of Physics and Technology, Key Lab of Artificial Micro- and Nano-Structures of Ministry of Education, Wuhan University, Wuhan 430072, People’s Republic of China 2 College of Physical Science and Technology, Guangxi University, Nanning, Guangxi 530004, People’s Republic of China 3 School of Electronic and Electrical Engineering, Wuhan Textile University, Wuhan 430073, People’s Republic of China 4 Institute of Nanoscience and Nanotechnology, Department of Physics, Huazhong Normal University, Wuhan 430079, People’s Republic of China 1. Experimental supplementary of ZnO quantum dots (QDs) synthesis ZnO QDs were synthesized via a low-cost solution method using Zn(CH3COO)2·2H2O (99.0% purity) and NaOH (96.0% purity) as the precursors1. Generally, the reaction can be described as follows: Zn(CH3COO)2 + 2NaOH → ZnO + H2O + 2NaCH3COO (1) Zn(CH3COO)2 and NaOH provided the Zn2+ and OH- for the synthesis of ZnO QDs , respectively. 1 For preparing Zn2+ solution, 150 mg Zn(CH3COO)2·2H2O was dissolved in 70 mL absolute ethanol (99.5% purity) (denoted as Solution 1). For preparing OH- solution, 10 mL of 1 M/L NaOH solution (complete dissolution of 1.6 g NaOH in 40 mL deionized water) was mixed with 40 mL absolute ethanol (denoted as Solution 2). Both Solution 1 and 2 were stirred in a 40 oC water bath constantly for 30 min to insure the complete and uniform dissolution of the Zn(CH3COO)2 and NaOH precursors. Thereafter, 5 mL of Solution 2 was added into Solution 1 drop by drop with stirring continuously and the molar ratio of Zn2+/OH- was fixed at 1.4.1 After adding Solution 2, the mixture was stirred for another 10 min and then transferred to a pre-heated oven at 40 oC for 4 h to promote the crystal growth of the ZnO QDs. The opalescent solution containing ZnO QDs was centrifuged and the obtained ZnO QDs were carefully washed with absolute ethanol for several times to remove the residual ions. After drying at 60 oC, a ZnO QD powder was obtained and measured to be ~6 mg. It should be noted that the ZnO QDs were not dried but redissolved ultrasonically in 6 mL absolute ethanol to obtain a ~1 mg/mL ZnO QD solution for the spin-casting process in device fabrication. 2. X-ray diffraction (XRD) of the ZnO QDs Supplementary Fig. S1 presents the XRD patterns of the obtained ZnO QD powders. All the diffraction peaks agree very well with the typical diffraction patterns of the hexagonal wurtzite ZnO (ICDD 36-1451). No diffraction peaks from impurities or contaminations are detected according to Supplementary Fig. S1. The average crystal size D can be determined from the diffraction peak widths and the diffraction angles by using the Scherrer equation: D 0.89 cos (2) where λ is the wavelength of the X ray (λ=0.154 nm), β is the full width at half maximum (FWHM) of the diffraction peak and θ is the corresponding Bragg angle of the diffraction peak. The average crystal size of the ZnO QDs is calculated with 2 equation 2 using XRD peaks of (100) and (101) and determined to be 6.1 nm, which is very consistent with the size distribution data in Fig. 1(d) (~5.9 nm). The slight difference might stem from the measuring errors and/or from the characteristics of diffraction and microscope methods.2 Supplementary Fig. S1. XRD patterns of the ZnO QDs with the Bragg angles ranging from 20 to 80o. Supplementary References 1. T. Toyama, H. Takeuchi, D. Yamaguchi, H. Kawasaki, K. Itatani and H. Okamoto, J. Appl. Phys. 108, 084302 (2010). 2. E. Erdem, J. Alloy Compd. 605, 34 (2014). 3