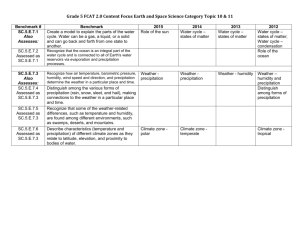
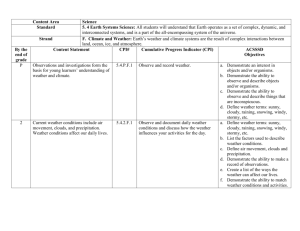
Study Buddy Unit II- Constant Velocity
advertisement

Study Buddy: Classification of Chemical Reactions Terms Law of Conservation of Mass Precipitation Reaction Definitions During a chemical or physical change, mass is conserved. Mass is neither created nor destroyed. Mass of reactants equals mass of products. Occurs when two aqueous solutions react to produce a solid precipitate. Representation N2 + 3H2 2NH3 N2 28g 2 atoms 3H2 6g 6 atoms 2NH3 34g 8 atoms NaCl(aq) + AgNO3(aq) AgCl(s) + NaNO3(aq) Mg + 2HCl Occurs when one or more electrons is MgCl2 + H2 transferred during a chemical reaction. Reduction: occurs when a species gains an electron 2H+ + 2e- H2 Oxidation: occurs when a species loses an electron Mg Mg2+ + 2e- Redox Reaction Representations: 1. Balance the following chemical equations and draw a particle diagram showing conservation of mass: a. Hydrogen gas and oxygen gas react to form liquid water. 2H2(g) + O2(g) 2H2O(l) b. Solid zinc is added to an aqueous solution of silver nitrate to produce zinc nitrate and solid silver. 2AgNO3(aq) + Zn(s) Zn(NO3)2(aq) + 2Ag(s) + NO3- + NO3- NO3- 2+ NO3- 2. A chemist added a 5.0g piece of solid magnesium metal to 10.0g of hydrochloric acid (HCl(aq)) in a 50 mL beaker. During the reaction, the solution in the beaker became warmer and produced many bubbles. Once the reaction finished, the mass of the final solution was only 14.6g. What is the best explanation for the “missing” mass? A bubbles are an indicator that a gas is being produced. In this case, 0.4g of gas were produced and no longer in the solution. 0.21 mole of Mg added to excess HCl produces 0.21 moles of H2(g) or 0.4g of H2(g) 3. Describe how you would make 100. mL of 2.0M NaNO3 from powdered NaNO3. In the box, show what happens to the NaNO3 when it is added to water. 1. Determine mass of NaNO3 needed: 2.0M = (X g/85.0 g/mol)/0.100 L X = 17g NO3- 2. Put 17g of NaNO3 in a 100 mL volumetric flask Na+ NO3- Na+ 3. Add water to 100 mL Na+ 4. Mix NO3- *water molecules not shown for clarity of ions 2/19/15 SCIE_CHEM_CLASSRXN_MAT_STUDYBUDDYTE_AL copyright © 2015 CFISD 1 4. Given the following experimental set-up, draw representations of the solutions in the boxes below. Beaker A 100.0 mL of NaCl(aq) Beaker B Beaker C A B Cl+ Na + Na Na+ Cl- 100.0 mL of AgNO3(aq) Beaker A mixed with Beaker B Cl- C NO3- Ag NO3- NO3+ Ag NO3- Na+ Ag+ + Na+ Na+ NO3- NO3AgCl *water molecules not shown for clarity of ions Write a balanced equation for the reaction. NaCl(aq) + AgNO3(aq) NaNO3(aq) + AgCl(s) What type of reaction occurred when A was mixed with B? precipitation 5. When a strip of zinc metal (shiny, silvery appearance) is placed in an aqueous solution of copper(II) sulfate, copper (brownish red appearance) will be deposited on the surface of the zinc. The reaction that occurs is shown below. Zn(s) + CuSO4(aq) ZnSO4(aq) + Cu(s) a. What species is being oxidized? __Zn__ b. What species is being reduced? __Cu2+_ c. Describe what is happening during this reaction in terms of electron transfer. Two electrons are being transferred to the Cu2+ that is in solution from Zn. When the electrons combine with the Cu2+ it forms solid Cu and leaves Zn2+ in solution with SO42- 6. In order to protect parts of a cargo ship from rusting as it floats in salt water, manufacturers bolt a piece of metal called a sacrificial anode to the side of the ship hull. Since the ship is made from steel, which is mostly iron, choose a good metal to make the sacrificial anode and explain your choice. Any metal that is above iron on the activity series chart will corrode before the iron corrodes. Elements that are higher on the chart are more easily oxidized thus protecting the iron hull from oxidation. Zinc is most commonly used. 7. Balance the following reactions and then identify as oxidation-reduction (OR), precipitation (P) or no reaction will occur (NR) and why. OR, P, NR a. 3N2(g) + 2H2(g) 2NH3(g) OR b. ___NaNO3(aq) + ___KCl(aq) ___KNO3(aq) + ___NaCl(aq) NR, both soluble c. ___BaCl2(aq) + ___Na2SO4(aq) ___BaSO4(s) + 2NaCl(aq ) P d. ___CH4(g) + 2O2(g) ___CO2(g) + 2H2O(g) OR e. ___Ag(s) + ___LiCl(aq) ___AgCl(s) + ___Li(s) NR, Ag less active than Li 8. Describe differences between redox and precipitation reactions in terms of the changes in the chemical equations and particle model. Redox reactions will have changes in the charges of the atoms/ions whereas all of the ions in a precipitation reaction remain the same. See question #1b for the particle model of a redox reaction; notice that the charges change for silver and zinc from reactant to product. See question #4 for the particle model of a precipitation reaction; the ions are rearranged, but unchanged. 2/19/15 SCIE_CHEM_CLASSRXN_MAT_STUDYBUDDYTE_AL copyright © 2015 CFISD 2 Unit: Classification of Chemical Reactions Planning Considerations Content: Unit side-by-side (show spiraling) Unit 1 (PROP) Identify chemical changes Intensive properties Concept of the mole Calculate number of ion, molecules or particles using the mole Unit 4 (SUBMIX) Solubility Molarity Differentiate between saturated and unsaturated Unit 6 (ATOMIC) Atomic structure Ion formation Unit 8 (PTABLE) Properties of chemical families Unit 9 (BOND) Ionic vs. Covalent Oxidation numbers Electrolytes Unit 10 (FORM) Writing formulas and naming compounds Unit 11 (ENRXN) Identify a reaction as endothermic or exothermic Possible flow – conceptual development flow rationale Patterns and observations of chemical reactions Representations of chemical reactions Precipitation reactions Redox reactions As with the previous unit (ENRXN), the unit begins with students observing chemical reactions and identifying observed macroscopic patterns (e.g. some combinations react, some don’t, some form precipitates, some bubble). The students should be exposed to a variety of chemical reactions that show the evidences of chemical change. 2/19/15 SCIE_CHEM_CLASSRXN_MAT_STUDYBUDDYTE_AL copyright © 2015 CFISD 3 Throughout the unit, the student should be exposed to describing chemical reactions in writing, pictures, and symbolically so that he/she can translate between the multiple representations as shown in question #1 of the Study Buddy. The traditional types of reactions (single replacement, double replacement, synthesis, decomposition, and combustion) should not be the focus of this classification unit. TEK C.10(H) says that a student should be able to “understand and differentiate among acid-base reactions, precipitation reactions, and oxidationreduction reactions.” Since we will conclude the year with acid-base reaction unit, we will concentration on precipitation reactions and redox reactions in this unit. The traditional classification types should be used within this structure. Students can use the traditional classification to predict the products of reactions, but the focus of the learning should be on the bonds being broken and formed. For example, when a student sees a reaction with the pattern AB + CD AD + CB, the student should be able to: 1. Describe how he/she knows that a reaction is taking place. 2. Are electrons being transferred? 3. Is a precipitate being formed? 4. Identify the reaction as precipitation, oxidation-reduction or neither. 5. What are the products of this reaction? 6. Draw a picture depicting the change. Although identifying the pattern as double replacement can be used to classify the reaction, it is not the skill that we are looking for. For precipitation reactions, the student should be able to describe what the aqueous reactants look like on the particulate level before and after the two aqueous solutions are combined. They should also verify their particulate model makes sense with the laboratory investigations and solubility of compounds in the STAAR Chemistry Reference Materials. This is a good time to make sure students remember that ionic compounds dissociate in water and covalent compounds do not and that this is related to the properties of these solutions as electrolytes and non-electrolytes, respectively. When given a double replacement reaction, the student should use the STAAR Chemistry Reference Materials solubility rules to determine if a precipitate will form. For oxidation-reduction reactions, the student should be able to describe the movement of the electrons in pictures, words and half-reactions. Using the rules for determining oxidation numbers, students should look at single replacement, synthesis and decomposition reactions to determine what species is oxidized and what species is reduced based on the changing oxidation numbers. Students will use the Activity Series of Metals on the STAAR Chemistry Reference Materials to determine if a replacement reaction will take place. We limit the activity of nonmetals to the halogens and their activity decreasing down the group. Students do not need to distinguish between oxidation number and charge at this level and are used interchangeably. Math/algebra Molarity Convert between moles, mass, and number of particles 2/19/15 SCIE_CHEM_CLASSRXN_MAT_STUDYBUDDYTE_AL copyright © 2015 CFISD 4 Misconceptions: Losing an electron results in a negative charge (oxidation number) while gaining an electron results in a positive charge. Students may want to change the subscripts instead of the coefficients to balance WEB resources: Classroom Resources (Can be used in the classroom for instruction) PheT o Balancing Chemical Reactions o Salts and Solubility o Molarity Gizmos o Balancing Chemical Reactions o Chemical Equations Pearson o Building Better Bridges Untamed Science Video Beautiful Chemical Reactions – (https://www.youtube.com/watch?v=BGUfC3UUBkI) This video shows close-up, slow motion precipitation reactions. Student Resources (Students can use for review at home) Pearson o Balancing Equations Student Tutorial Video o Double-Replacement Reaction Equation Student Tutorial o Solubility Rules Pearson Flipped Video for Science o Classifying Chemical Reactions Pearson Flipped Video for Science YouTube: o A Beginner’s Guide to Balancing Equations (https://www.youtube.com/watch?v=_B735turDoM) o Precipitation Reactions: Crash Course Chemistry #9 (https://www.youtube.com/watch?v=IIu16dy3ThI) o Redox Reactions: Crash Course Chemistry #10 (https://www.youtube.com/watch?v=lQ6FBA1HM3s) o Redox Reactions (https://www.youtube.com/watch?v=RX6rh-eeflM) o How to Use Solubility Rules to Predict Precipitate Reactions (https://www.youtube.com/watch?v=2XRGrBvQTU8) POGIL: Flinn Scientific POGIL Activities for High School Chemistry (2012) Types of Chemical Reactions; Page 153 Oxidation and Reduction; Page 267 The Activity Series; Page 275 2/19/15 SCIE_CHEM_CLASSRXN_MAT_STUDYBUDDYTE_AL copyright © 2015 CFISD 5 PD Support: Team meetings, Share Sessions Released STAAR/EOC Questions: 8, 13, 19, 35, 43, 46 8th Grade STAAR Questions: 2013 – 18 2013 – 23 2/19/15 SCIE_CHEM_CLASSRXN_MAT_STUDYBUDDYTE_AL copyright © 2015 CFISD 6 2014 – 10 2014 – 25 2/19/15 SCIE_CHEM_CLASSRXN_MAT_STUDYBUDDYTE_AL copyright © 2015 CFISD 7 2014 – 31 2014 – 39 2014 – 44 2/19/15 SCIE_CHEM_CLASSRXN_MAT_STUDYBUDDYTE_AL copyright © 2015 CFISD 8