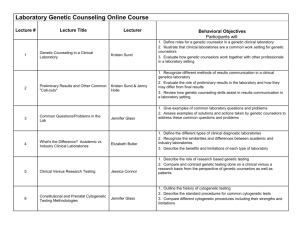
ExomeNext Letter of Medical Necessity
advertisement

LETTER OF MEDICAL NECESSITY FOR CLINICAL EXOME SEQUENCING (ExomeNext) Date: Date of service/claim To: Utilization Review Department Insurance Company Name, Address, City, State Re: Patient Name, DOB, ID # ICD-9 Codes: (list codes) This letter is in regards to my patient and your subscriber, First, Last Name, requesting full coverage of medically-indicated genetic testing for clinical exome sequencing to be performed by Ambry Genetics Corporation (TIN 33-0892453 / NPI 1861568784), a CAP-approved and CLIA-certified laboratory located at 15 Argonaut, Aliso Viejo, CA 92656. Approximately 85% of genetic changes that cause known diseases occur within the exome – the set of protein-coding regions that is about 1-2% of the entire human genome.1 Whole exome sequencing has been shown to be a successful option for diagnosing individuals with previously unidentified genetic conditions.2 The American College of Medical Genetics and Genomics published a 2012 Policy Statement on the clinical application of genomic sequencing.3 The statement recommends exome sequencing for the following clinical scenarios (adapted from original publication): The patient’s clinical presentation (phenotype) and family history strongly implicate a genetic etiology, but phenotype does not correspond with a specific disorder for which a clinical targeted genetic test is available Clinical presentation (including fetal, with limitations) suggests a likely genetic disorder, but specific genetic tests (including targeted sequencing tests) for phenotype have failed to provide a diagnosis A defined genetic disorder with a high degree of genetic heterogeneity is suspected, making whole exome or genome sequencing of multiple genes simultaneously a more practical approach Significant aspects of my patient’s personal and/or family medical history that raise reasonable suspicion of an underlying genetic diagnosis are as follows: Based on the above, I suspect my patient could have: Syndrome X (for which a genetic etiology is highly suspected, but yet to be discovered) In order to evaluate for the above conditions, my patient has had the following uninformative tests: Due to the heterogeneous nature of my patient’s symptoms and uninformative test results thus far, there is a reasonable probability of detecting a genetic mutation using this test. Per ACMG guidelines, clinical exome sequencing is warranted for my patient.3 This test (ExomeNext) thoroughly analyzes the exome and mitochondrial genome, with extensive results analysis and interpretation. I am choosing ExomeNext because of its diagnostic rates as compared to other options in the industry,4 and because is a highly efficient and cost-effective way to analyze the functionally relevant regions in our 20,000 genes. Clinical exome sequencing has a significant likelihood of providing my patient and his/her family with an accurate diagnosis, thereby ensuring appropriate medical management. In many cases, a diagnosis can lead to a specific treatment or management strategy that dramatically changes the clinical outcome.2,5 A diagnosis can also help identify necessary medical referrals, screening for associated complications, and recurrence risk counseling.2,5 Once a diagnosis is made, the costs associated with further testing are likely to decrease.2,5 Due to these benefits, exome sequencing is medically warranted in my patient. As such, I am ordering this medically necessary test and affirm that my patient has provided informed consent for genetic testing. A positive test result would confirm a genetic diagnosis and/or risk in my patient, and would ensure my patient is being managed appropriately. I am specifying Ambry Genetics Corporation because this laboratory was the first to commercially offer clinical exome sequencing, and does so in a highly-sensitive and cost-effective manner. It also has a large database of tested patients to ensure highly validated, accurate, and informative test interpretation. I recommend that you support this request for coverage of clinical exome sequencing in my patient. Depending on the exact test ordered, genetic testing can take up to several months to complete and the laboratory will not bill until testing is concluded. Therefore, we are requesting that the authorization be valid for 12 months. Thank you for your time and please don’t hesitate to contact me with any questions. Sincerely, Ordering Clinician Name (Signature Provided on Test Requisition Form) (MD/DO, Clinical Nurse Specialist, Nurse-Midwives, Nurse Practitioner, Physician Assistant, Genetic Counselor*) *Authorized clinician requirements vary by state Test Details CPT codes: 81404x2, 81405x2, 81406x2, 81407x2, 81408x2 Laboratory: Ambry Genetics Corporation (TIN 33-0892453 / NPI 1861568784), a CAP-accredited and CLIA-certified laboratory located at 15 Argonaut, Aliso Viejo, CA 92656 References: 1. 2. 3. 4. 5. Pussegoda KA. Exome sequencing: locating causative genes in rare disorders. Clin Genet. 2010 Jul;78(1):32-3. Biesecker LG and Green RC. Diagnostic clinical genome and exome sequencing. N Engl J Med. 2014;370:2418-25. ACMG Board of Directors. ACMG Policy Statement: Points to consider in the clinical application of genomic sequencing. Genet Med. 2012;14(8):759-76. Farwell KD, et al. Enhanced utility of family-centered diagnostic exome sequencing with inheritance model-based analysis: results from 500 unselected families with undiagnosed genetic conditions. Genet Med. 2014 Nov 6. [Epub ahead of print] Iglesias A, et al. The usefulness of whole-exome sequencing in routine clinical practice. Genet Med. 2014;16:922-931.