WS Atomic Structure
advertisement

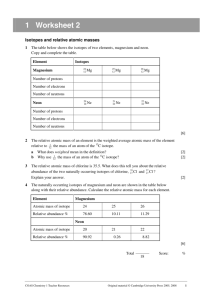
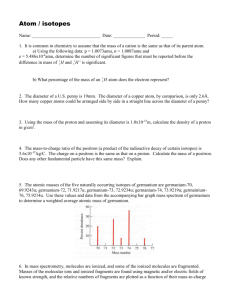
WS Atomic Structure (Atoms, Isotopes, & Mass Spectrometry) 1. What is the charge on a sodium atom? 2. What is the charge on a sodium nucleus? 3. What is the atomic number of potassium? 4. How many protons are there in the nucleus of a potassium atom? 5. How many electrons in the potassium nucleus? 6. What is the most likely charge (the most common charge) on an ion of sulfur? 7. If a chloride ion and a strontium ion were to form a compound, what would its formula be? 8. What do all the ions of the transition metals have in common with one another? 9. Many elements have a number of isotopes. (a) Complete the following table. (b) Consider the 2nd and 4th row in the table. What three things do the isotopes have in common? (c) Consider the 2nd and 4th row in the table. What two things are different between the isotopes? (d) Define the term isotope. 1 10. The diagram below represents the spectrum of chlorine gas, consisting of five peaks, labeled I, II, III, IV, and V respectively. Peak I is due to the 35Cl+ ion. (a) What is the analytical technique (or instrument) used to give a spectrum like to one shown? (1) (b) The spectrum shows peaks I and II at 35.00 and 37.00. Given that the atomic relative abundances of Cl 35.00 and Cl 37.00 are 77.50% and 22.50% respectively, calculate the average relative atomic mass of chlorine atoms to four significant figures. (2) (c) Consider why the spectrum of chlorine gas consists of more than just the two peaks in part (b). Explain each of the other three peaks (III, IV, and V) occurring at 70.00, 72.00, and 74.00. (d) If peaks III, IV, and V were not shown, how could you have used peaks I and II to predict which molecule of Cl2 would be most abundant in nature? 2 11. The average atomic mass of naturally occurring neon is 20.18 amu. There are two common isotopes of naturally occurring neon as indicated in the table below. (2007B #2) Isotope Mass (amu) Ne-20 19.99 Ne-22 21.99 (i) Using the information in the table above, calculate the percent abundance of each isotope. (1) (ii) Calculate the number of Ne-22 atoms in a 12.55 g sample of naturally occurring neon. (3) 3