U8 Review WS 2015
advertisement

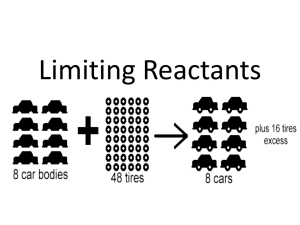
U8 Test Review A: Stoichiometry Name ______________ Period _____ For this unit test, you will need to be able to: explain what happens during a chemical reaction. complete a BCA table in order to determine how much of each reactant is used up and/or how much product is formed. complete a BCA table to determine the theoretical yield of a product & then calculate the percent yield when given an actual yield. determine the limiting reactant, which reactant is in excess, and how much excess is left when given amounts of each reactant involved in a reaction. determine a balanced equation, a theoretical yield, an actual yield, and a percent yield based on lab data. 1. Nitrogen gas reacts with hydrogen gas to form ammonia gas, or NH3. Write the balanced equation below: a. True or False: The balanced equation shows the number of atoms is conserved. b. True or False: The balanced equation shows the number of molecules is conserved. c. Given the picture below where the striped circles = nitrogen, and the open circles = hydrogen, draw what the resulting products would look like. d. What is the limiting reactant? How do you know this? e. How many excess reactant molecules are left? 2. Nitrogen monoxide reacts with oxygen gas to produce nitrogen dioxide. If 0.66 g of nitrogen monoxide were reacted excess oxygen, how many grams of nitrogen dioxide would be formed? Complete a BCA table. Make sure to label all units & identify your answer. 2. Phosphorus trichloride reacts with water to produce phosphorous acid, H3PO3 and hydrochloric acid, HCl. How many moles are phosphorus trichloride are needed to react completely with 123g of water? Percent Yield 3. Aluminum metal reacts with copper (II) sulfate to produce solid copper and aqueous aluminum sulfate. If 1.85 g of Aluminum is reacted with excess copper (II) sulfate, what is the theoretical yield of copper. If you actually obtain 10.8 g of copper, what is your percent yield? Limiting Reactants 4. Hydrazine, N2H4 , reacts with dinitrogen tetroxide to produce nitrogen gas and water. If 10.5 g of hydrazine react with 12.4 g of dinitrogen tetroxide, what is the limiting reactant? How many grams of the excess reactant will be left over? Lab data 5. A student places a piece of magnesium ribbon into lead (II) nitrate. Lead metal and aqueous magnesium nitrate were produced. A large strip of magnesium ribbon was placed into the lead (II) nitrate solution, and left to react overnight. The following day, there was still some strip of magnesium left, and the following data was collected: Data: Mass of beaker: 108.55 g Mass of beaker + lead: 130.15 g Mass of magnesium before reaction: 6.50 g Mass of magnesium after reaction: 3.86 g Mass of lead produced ____ Mass of magnesium used ____ Moles of magnesium used in reaction: WORK: Balanced Equation: BCA Table: Show all proportions except for 1:1 proportions. 4 decimal places for moles! Theoretical (BCA) yield of lead (g): show work % yield of lead: Actual (data) yield of lead: Show your work! U8 Test Review B: Stoichiometry Name ______________ Period _____ For this unit test, you will need to be able to: explain what happens during a chemical reaction. complete a BCA table in order to determine how much of each reactant is used up and/or how much product is formed. complete a BCA table to determine the theoretical yield of a product & then calculate the percent yield when given an actual yield. determine the limiting reactant, which reactant is in excess, and how much excess is left when given amounts of each reactant involved in a reaction. determine a balanced equation, a theoretical yield, an actual yield, and a percent yield based on lab data. 1. In the reaction between phosphorous and oxygen, phosphorous trioxide is produced. Balanced equation: a. True or False: The balanced equation shows the number of atoms is conserved. b. True or False: The balanced equation shows the number of molecules is conserved. c. If 12 phosphorous atoms and 9 oxygen molecules react, how many molecules of phosphorous trioxide can be produced? d. What is the limiting reagent? How do you know this? e. What is the excess reagent? How much excess reagent is left over? f. a. Balance this rxn: barium phosphate reacts with sodium sulfate to produce sodium phosphate and barium sulfate. g. If 25.0 grams of barium phosphate react with 65.5 g of sodium sulfate, and 41.8g of sodium phosphate is produced. Barium sulfate is also a product. How much mass of barium sulfate must be produced? h. What Law is this demonstrating? i. What else is conserved(same before and after a rxn) in any chemical rxn? Circle all that apply. atoms molecules moles j. In the reaction between calcium phosphite and silver chromate, calcium chromate and silver phosphite are produced. If 100. grams of each reactant reacts, how much silver phosphite can be produced? Calculate the moles and the mass. If 50.02 g are actually produced, what is the % yield? How many molecules can be produced? k. . If 1025 g of chlorine and excess calcium iodide react, calcium chloride and iodine are produced. How much calcium iodide would be used to completely react with the chlorine? Lab data A student placed a piece of aluminum in copper(I)nitrate solution. Copper metal precipitated and aqueous aluminum nitrate was produced. The following day, there was still some of the aluminum left, and the following data was collected: Data: Mass of beaker Mass of beaker + copper Mass of aluminum before reaction Mass of aluminum after reaction Mass of copper produced Mass of aluminum used Moles of aluminum used in reaction: 125.9g 132.2g 3.85g 2.89g ____ ____ WORK: Balanced Equation: BCA Table: Show all proportions except for 1:1 proportions. 4 decimal places for moles! Theoretical yield of copper: Show your work! % yield of copper: Actual yield of copper: Show your work!