SCH3U Limiting Reagent and Percent Yield ppt

SCH 3U1
2
Review
In a chemical reaction, how do we relate moles of one compound to moles of another?
2A + B A
2
B
MOLE RATIO!
What are the four steps to go from mass of one reactant or product to the mass of another?
1.
Write the balanced equation
2.
Convert mass of given to moles
3.
Use the mole ratio from the balanced equation to find moles of the unknown
4.
Convert moles of the unknown to mass of the unknown
3
Chemical Construction
In your group, you have a beaker of hydrogen (H
2
) and a beaker of carbon
You need to make as many methane molecules (CH
4 you can!
) as
4
Activity Analysis
Which element limited the number of methane molecules you could make?
Which element was present in excess amounts?
Did the element present in excess affect the number of methane molecules you could make?
Why doesn’t the carbon limit the number of methane molecules you could make, even though they are present in the smallest quantity?
5
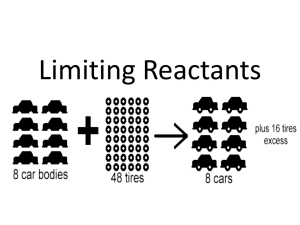
Limiting Reactant
Reactants are rarely present in amounts that correspond exactly to the mole ratios in the balanced equation
Limiting reactant: a reactant that is completely
consumed during a chemical reaction and limits the amount of product that can be formed
Excess reactant: a reactant that remains after a reaction is over
Limiting Reactant
6
L.R.
2 mol
2 mol
4 mol
1 mol
2 mol
1 mol L.R.
2 mol
The limiting reactant forms the smallest amount of product
Use stoichiometry to determine which reactant produces the smallest amount of product
7
Practice Problem
Lithium nitride reacts with water to form ammonia and lithium hydroxide, according to the following balanced chemical equation:
If 4.87 g of lithium nitride reacts with 5.80 g of water, find the limiting reactant.
8
Yield
Theoretical yield is the maximum amount of product that can form in a chemical reaction
Calculated by assuming that all of the limiting reagent has reacted to form the product
In reality:
Many reactions do not go to completion
What are some things that might prevent us
Other reactions, called side reactions , may occur
Some product may be lost during purification
9
Yield
The actual yield is the amount of a product that is actually obtained from a chemical reaction
The actual yield is almost always less than the theoretical yield
Actual Yield is an experimentally determined quantity
Percent yield:
% Yield = actual yield x 100 theoretical yield
Crucial Information for Chemists
Consider the synthesis of Diazonamide A
82% 95% industrial chemistry involves many steps
88%
all yield
88%
resources
67%
80%
10
50%
60%
R. R. Knowles, J. Carpenter, S. B. Blakey, A. Kayano, I. K. Mangion, C. J. Sinz, D. W. C. MacMillan, Chem. Sci., 2011, 2, 308-311
11
Practice Problem
Ammonia can be prepared by reacting nitrogen gas with hydrogen gas:
When 7.5 x 10 1 g of nitrogen reacts with sufficient hydrogen, the theoretical yield of ammonia is 9.10g. If 1.72 g of ammonia is obtained by experiment, what is the percentage yield of this reaction?
12
Homework
Practice Problems