Water and Solutions Cornell Notes PAP
advertisement
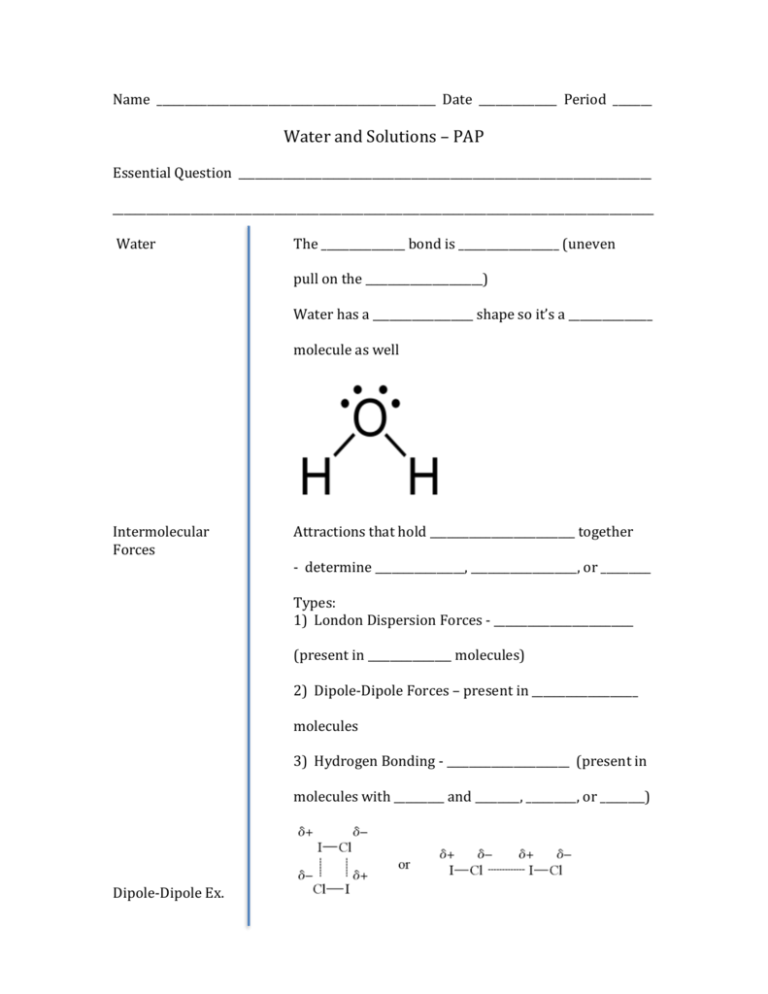
Name __________________________________________________ Date ______________ Period _______ Water and Solutions – PAP Essential Question __________________________________________________________________________ _________________________________________________________________________________________________ Water The _______________ bond is __________________ (uneven pull on the _____________________) Water has a __________________ shape so it’s a _______________ molecule as well Intermolecular Forces Attractions that hold __________________________ together - determine ________________, ___________________, or _________ Types: 1) London Dispersion Forces - _________________________ (present in _______________ molecules) 2) Dipole-Dipole Forces – present in ___________________ molecules 3) Hydrogen Bonding - ______________________ (present in molecules with _________ and ________, _________, or ________) Dipole-Dipole Ex. Hydrogen Bonding in Water - Molecules containing these bonds show ______________ strong ___________________________ to one another – keeps the molecules ________________________________________ - This is the reason for ________________ being a ____________ at __________________ temperature - Normally molecules with __________________ masses have ________________ intermolecular forces and therefore are ______________________ - but NOT ______________________ Ice Most things ________________________ as they cool Water __________________________ until _________________, then the _______________________ starts to __________________ - Large, ______________________ rings of ice crystals form - Increase in __________________, so density _________ - Reason that ice __________________ in water Essential Question __________________________________________________________________________ _________________________________________________________________________________________________ Aqueous Solutions Solute – what is __________________________________________ Water is the __________________________ - it is doing the _____________________________ Ionic and polar molecules ______________________ in water Nonpolar molecules __________________ “like ________________________ like” WHY? Water is ______________________ It has partially __________________ and _________________ ends Its __________________________ to other __________________ which _____________________________ and other _______________ molecules have - Water will ________________ anything else with ___________ The __________ of one is attracted to the _________ of other Water can _______________ ionic compounds apart and Then _____________________ the ions to _________________ them Electrolytes Conducting electricity requires ____________________, ____________________ particles Ionic compounds that __________________ in water produce ____________________ and are called ___________________________ * Reminder – ions are ___________________ particles When electricity is passed through the ___________________, these ______________ carry electricity through the solution More ______________ means __________________ conductor Ex. Which is a better conductor: AlCl3 or (NH4)2CO3? - Most molecular solutions do not split into ____________ and therefore do not ___________________ electricity – they are called _______________________________ Heterogeneous Aqueous Mixtures Suspensions – mixtures that _________________ out upon standing - particles are too ________________ to fully dissolve - they can be ________________ - particles can be _____________________ out Examples: Colloids – mixture with __________________-sized particles - particles are too ________________ to fully dissolve, but too ___________________ to see - Look ___________________ or _________________ - Cannot _____________________ out particles Examples: Tyndall Effect – both suspensions and colloids - scattering of ___________________ in all directions - particles have to be _________________ enough to reflect ________________ - Solutions ________________ exhibit Tyndall Effect - particles are too ___________________ Essential Question __________________________________________________________________________ _________________________________________________________________________________________________ Solution Formation Soluble – substance can _____________________ Ex. Sugar in water Insoluble – substance __________________ dissolve Ex. Sand in water Rate of Solution Formation Factors that Increase Rate 1) Stirring/Agitating - It moves the __________________________________ away - It brings fresh _________________ in contact with the _________________ to dissolve more 2) Increasing Temperature - Increases the _____________________ of the water and _______________ particles causing them to come in contact _______________ frequently 3) Increasing Surface Area - Dissolving takes place from the ____________________ Solubility Solubility is the _______________________ amount of solute that can ______________________ in a specific amount of _______________________ at a specific ___________________________ Types of Solutions 1) Saturated – contains the ______________________ amount of dissolved ______________________ (can’t dissolve __________ at that ________________________________) 2) Unsaturated – contains _______________ than the __________________________ amount of _________________ 3) Supersaturated – contains ________________ than the _______________________ amount of ____________________; - an ________________________ solution To prepare: - Add _________________ solute to a ______________________ solution; __________________ it up until the __________________ solute dissolves; let it ______________ back to the original ______________________________ - disturbing the __________________________ can cause the extra _________________ to “_____________________________” of solution (______________________________ out) Solubility Curves Points that fall ____________ the line - ________________________ Points that are ___________ the line - ________________________ Points that are ____________ the line - ________________________ Examples: 1) What type of solution is formed from 40 g of KNO3 at 50oC? 2) At what temperature would 30 g of KClO3 form a saturated solution? 3) How many grams of Pb(NO3)2 can dissolve in 200 g of water at 40oC? 4) If a saturated solution of KCl is cooled fro 80oC to 30oC, how many grams of KCl will precipitate out? Factors Affecting Solubility Solids in Liquids - Temperature: most __________________ will be ____________ soluble at ______________________ temperatures Gases in Liquids - Temperature: Gases become ________________ soluble at _______________________ temperatures because the gas particles have _________________ energy to ___________________ the top of the liquid and ___________________________ - Pressure: Gases become ________________ soluble as the pressure above it ________________________ Essential Question __________________________________________________________________________ _________________________________________________________________________________________________ Concentration Solutions can be described: 1) Qualitatively: - Concentrated - there is ______________________ of solute - Dilute – there is a _____________________________ of solute 2) Quantitatively: Equation: Molarity (M) = It’s shown with the _____________________ of a solution. Ex. 1.5 M NaCl (aq) is described as “1.5 molar” Examples 1) What is the molarity when 2.0 moles of glucose is dissolved in 5.0 L of solution? 2) How many grams of Na2SO4 would be required to make 1.5 L of 0.24 M solution? Dilutions Can start with a solution of ______________ concentration and add __________________ to ____________________ the concentration Equation Example What volume of 2.00 M CaCl2 would be needed to make 0.50 L of 0.300 M CaCl2?