GI Accessory Organs - HistologyforMedStudents
advertisement

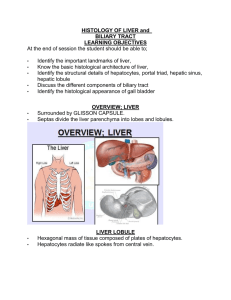
GI Accessory Organs Lab There are six slides to examine, all in the ‘folder’ on the Bacus site labeled “Liver, Gallbladder, and Pancreas”. Slides 57 and 58: Pancreas. The slides are very similar but the second one is a thin plastic section and, although pale-stained, shows more detail, especially of the exocrine acinar cells. Examine both slides, focusing first on the exocrine acini. Note the pieshaped arrangement of the secretory cells, with their basal basophilia, central nuclei, and apical (esosinophilic) secretory granules. You should be able to ‘predict’ what an electron micrograph of these cells would show in terms of organelle content and arrangement. You should also be able to state what is contained in the secretory granules, what controls their release (by exocrine, merocrine secretion), how the secretions reach the small intestine (where?) and what they do there. Locate and identify centroacinar cells. What are they? Compare the ductwork of the pancreas with that of the major salivary glands. Does the pancreas have striated ducts? Does it have mucous acini? Find examples of the (endocrine) Islets (of Langerhans). What cells do these contain and what do the cells produce? Are the Islets surrounded by a connective tissue capsule? How can you distinguish the Islets from the exocrine acini? Note, in slide 57, a sort of hilus, at which major blood vessels enter/leave the pancreas. Observe the adipose tissue. Can you find any nerve fibers? Lymphatic vessels? There are three liver slides. Slide 60: Liver, H&E. This is a fairly thin plastic section with quite decent preservation. The sinusoids are filled with blood (which is how they would be in life of course) but because individual RBCs are difficult to distinguish, the slide is a bit confusing. Working at high magnification, focus your attention on the intersecting rows (cords) of hepatocytes. These are the predominant cell type, with nice round, mostly-euchromatic (why?) nuclei. Although you cannot identify individual organelles, it is obvious that the cytoplasm of these cells has considerable substructure. What are these cells doing and what organelles would you expect to find in an electron micrograph? Identify the ‘endocrine faces’ of the cells (closest to the sinusoidal lining cells), and the ‘exocrine faces’. In many areas the bile canaliculi are remarkably well preserved and can be seen in both longitudinal and cross section. Most of the elongate (dark) nuclei belong to endothelial cells, lining the sinusoids. What kind of vessels are these sinusoids? It is unlikely that you will be able to identify the other cells associated with the sinusoids, but you should know what they are and what they do. Now go back down to lower magnification and find patches of connective tissue, the portal areas, in which blood vessels and ducts are found. Understand that it is rare for a single section of these portal tracts to show all the components that run there, but you should be able to find bile ducts, branches of the hepatic artery and portal vein. Look for lymphatics and small nerve bundles – these are much more rarely seen. In areas approximately equidistant from several portal tracts, try to find central veins (larger blood spaces than sinusoids, but with very thin linings. Because so much blood is packed in the lumens of the sinusoids, it is actually rather difficult to identify central veins in this slide. Central veins never have a lot of connective tissue around them and are never associated with bile ducts. Mentally trace the flow of blood, bile, and lymph in the liver. Slide 25: Lymph node and Liver, Reticulum stain. The liver specimen is the rectangular chunk to the right. Although cell preservation in this slide is much poorer than in slide 60, the staining of the network of reticular fibers (what class of collagen?) provides an excellent picture of the framework of the liver and the directions of fluid flow. First look at any area at the highest magnification and try to distinguish the very finest black fibers (reticular) from the somewhat thicker fibers (collagen, probably type I) that are stained a dark brown. Reticular fibers are found between hepatocytes and adjacent sinusoidal lining cells but never between two hepatocytes. Portal tracts have numerous reticular fibers and coarser collagen fibers. In this section the central veins are much easier to identify, partly because the staining of the reticular fibers of the sinusoids gives some sense of the overall direction of blood flow. But, in addition, where sinusoids converge on central veins, it is clear that the central veins have, in addition to the reticular fibers characteristic of the sinusoids, some brownish fibers (collagen) reflecting the fact that the central veins are larger than the sinusoids and are the beginning of the blood collecting vasculature. Slide 60A (Mallory-Azan) is awful – skip it. Slide 66 : Gallbladder. At low power note the lymphoid nodules in the wall of this organ. Another example of the widespread distribution of MALT. Scan around at intermediate magnifications, noting that the wall of this organ has a mucosa (epithelium and lamina propria) and a muscularis (bundles of smooth muscle) as well as a serosa, but there is no special layer of smooth muscle close to the mucosal folds that allows one to distinguish a mucosa/submucosa boundary. In other words, the Gallbladder wall has THREE layers, not the four layers of the digestive tract. Note the extensive folding of the mucosa, but be aware that none of the deep folds are glands even though the folding is so extensive that some of the pockets seem ‘separated’ from the surface epithelium. Next, working at the highest magnification, observe the mucosal epithelium. This is perhaps the ‘cleanest’ simple columnar epithelium in the body. All the cells are the same (except for the occasional lymphocyte traversing the epithelium) and all are doing the same thing: removing fluid from the bile (thus concentrating it), so that larger amounts can be stored until the action of CCK (from the GI enteroendocrine cells) stimulates the muscularis to contract, forcing the stored bile out of the gallbladder and into the common bile duct for release into the duodenum. Although the preservation is not good enough to reveal the extensive brush border (increased absorptive surface) or the junctional complexes (so bile does not leak between the epithelial cells), both are present.