AP Biology Name AP Biology Modeling with C, H and O In this
advertisement

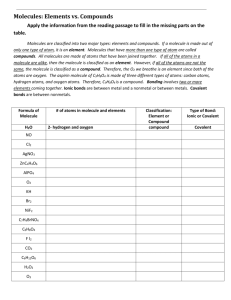
AP Biology Modeling with C, H and O In this exercise, you will explore the structures that can be constructed using only carbon, hydrogen and oxygen. These are the elements needed to build carbohydrates and lipids, and they are essential components of proteins and nucleic acids, along with nitrogen, phosphorus and a bit of sulfur. Make sketches of what your draw in your lab notebook and add notes about the molecule based on the questions below. Conventions: Black = C red = O white = H; one section of white straw = single covalent bond For ring structures, atoms that stick out toward you should be drawn as heading up from the carbon, while those that point away from you (towards the desk top) should be drawn as sticking down from the carbon. Carbohydrates 1. Start with 6 C, 12 H and 6 O, and connect these to form a linear or chain structure. You must use ALL of the atoms, but you are free to consider double bonds in addition to single. Rearrange the atoms to form two more structures. Sketch each structure on your answer sheet. 2. These structures all have the same chemical formula, but the atoms are arranged in different ways. How are these different shapes related? What are they called? Must you break a bond in order to rearrange the atoms from one form to another, or can you rotate atoms around a carbon? 3. Consult the reference sheet and try to identify each molecule. There are other possibilities, but the ones shown are the most common and most likely to be biologically active. 4. Build a linear molecule of glucose. Recall that in water, in other words in living things, the monosaccharides tend to form ring structures. Change your linear model into a biologically active ring form as shown below. Note that only atoms attached to carbons 1 and 5 should be involved. AP Biology 5. In the linear form, what functional group was visible? Where is it in the ring form? 6. The transition to a ring may produce two different forms of glucose, and as shown below. How are they different? The difference is subtle, but it has major implications. 7. Time to create a disaccharide! Build two molecules of glucose. These can be joined through a condensation reaction involving the hydroxyl groups attached to carbons 1 and 4. This is a 1,4 glycosidic linkage. Draw the products of this reaction on your answer sheet. What is this disaccharide called? 8. On to polysaccharides! Join your disaccharides to others in your class. As the chain elongates, what form does it take? Describe it. You are creating starch. 9. Now hydrolyze the linkages to recover your monomers. 10. Turn one of your -glucose forms into the form. Join these together using the 1, 4 linkage. You can do it, but it requires some thinking. Explain on your answer sheet. 11. Now join your disaccharide with others in the class to create cellulose. How does the structure of this chain differ from the starch? 12. Hydrolyze the bonds to recover your starting glucose molecules and then dismantle the structures. It’s time to repurpose your atoms as we consider lipids. AP Biology Lipids 1. The most common basic form of lipids that you should know is the triglyceride. It consists of one glycerol molecule bonded to three fatty acids. This is a macromolecule, but not a polymer. Explain why. 2. Build a glycerol: C3H8O3. It should contain three hydroxyl groups. Sketch it on your answer sheet. 3. Now build a short fatty acid. It must have a carboxyl group at one end and otherwise should only consist of a chain of 5 additional carbons and enough hydrogens to fill the carbons. Everything should be single bonded. Write the formula for this molecule. Is it hydrophobic or hydrophilic? Explain. 4. Join the fatty acid to the glycerol molecule using a condensation reaction. The carboxyl of the fatty acid should interact with a hydroxyl of the glycerol. Share with another group or two to create a full triglyceride. What byproduct has also been produced from the formation of each bond? 5. Now hydrolyze the bonds and take back your original molecules. Use the carbons and hydrogens from the glycerol to extend your hydrocarbon chain. The carbons are saturated with hydrogens. Modify this molecule so that is polyunsaturated – two double bonds should be enough. How has the structure of the fatty acid chain changed relative to when it was saturated? 6. Which form, saturated or unsaturated, would you be able to pack more densely? How does your answer relate to dietary fats? We consider lipids that are solid at room temperature to be fats, and those that remain liquid at room temperature to be oils. Which form would likely contain more unsaturated bonds? 7. If you wanted to use fatty acids as a component of a flexible barrier - like maybe a cell membrane – would saturated or unsaturated chains be a better bet? Explain. 8. Disassemble you model and put all the pieces back in the original plastic bags. AP Biology AP Biology Name AP Biology Modeling Carbohydrates and Lipids Carbohydrates 1. Sketch each of your three linear structures 2. These structures all have the same chemical formula, but the atoms are arranged in different ways. How are these different shapes related? What are they called? Must you break a bond in order to rearrange the atoms from one form to another, or can you rotate atoms around a carbon? 3. Consult the reference sheet and try to identify each molecule. Add these labels to the molecule sketches above. 5. In the linear form, what functional group was visible? Where is it in the ring form? 6. Draw the products of this reaction. What is this disaccharide called? 7. On to polysaccharides! As the chain elongates, what form does it take? Describe it. Name AP Biology 9. Turn one of your -glucose forms into the form. Join these together using the 1, 4 linkage. You can do it, but it requires some thinking. Explain on your answer sheet. 10. Now join your disaccharide with others in the class to create cellulose. How does the structure of this chain differ from the starch? Lipids 1. This is a macromolecule, but not a polymer. Explain why. 2. Build a glycerol: C3H8O3. It should contain three hydroxyl groups. Sketch it below. 3. Write the formula for this molecule. Is it hydrophobic or hydrophilic? Explain. 4. What byproduct has also been produced from the formation of each bond? 5. How has the structure of the fatty acid chain changed relative to when it was saturated? Name AP Biology 6. Which form, saturated or unsaturated, would you be able to pack more densely? How does your answer relate to dietary fats? We consider lipids that are solid at room temperature to be fats, and those that remain liquid at room temperature to be oils. Which form would likely contain more unsaturated bonds? 7. If you wanted to use fatty acids as a component of a flexible barrier - like maybe a cell membrane – would saturated or unsaturated chains be a better bet? Explain.