The system above is at equilibrium. What is meant by equilibrium
advertisement

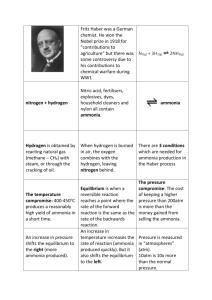
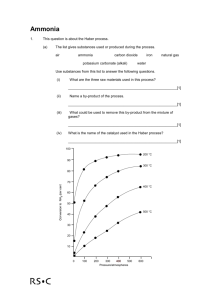
1. The system above is at equilibrium. What is meant by equilibrium? The numbers indicate that the final percent of ammonia is constant. The forward reaction is equal to the reverse reaction at this point. 2. The original process was too slow and produced low yields. Produce a well written paragraph explaining three factors that can be changed to speed up the reaction and increase the yield. Decreasing temperature will increase yield, Increasing pressure will increase yield, increasing concentration of the reactants will increase yield. A catalyst will speed up the reaction. Other possible responses- decrease volume. 3. Produce a correctly labeled and formatted graph of the data from table one on a separate piece of graph paper. Choose two of the temperatures to plot pressure versus ammonia yield data. X and y axis labeled with units, title, line graph, axes correctly numbered, points plotted for two temperatures, legend, size of graph is appropriate for the paper 4. If the condition are 200 atmospheres and 425 O C what yield of ammonia is obtained at equilibrium? Answer near 30 5. Pick out the conditions of temperature and pressure from those listed in Table 1 that would give the highest yield of ammonia. 100 degrees C and 400 atm 6. The forward reaction in the Haber Process is exothermic (∆Ho = -92 kJ mol-1). Explain how the experimental results in Table 1 are in agreement with Le Chatelier’s Principle. An increase in temperature shifts an exothermic reaction away from the products and toward the reacants. This reduces the yield of ammonia. 7. The boiling points N2, H2, and NH3 are -196 °C , -253 °C and -33 °C respectively. How do you think the ammonia could be separated from unreacted nitrogen and hydrogen? Cool below -33 and above -196 to make ammonia liquid while the reactants are in a gas state.