Atomic Structure & Nuclear Chemistry Study Guide
advertisement

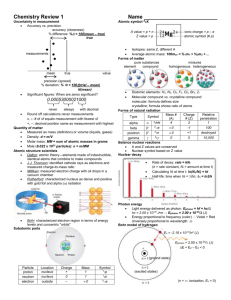
Study Buddy: Atomic Structure and Nuclear Chemistry Development of the Modern Atomic Theory Scientist Experiment No experiment Democritus Use experiments of previous scientists Dalton Cathode ray Thomson Gold foil Rutherford Alpha particles Chadwick Uses findings from previous scientists and Quantum theory Multiple experiments Bohr Modern Atom Findings Atoms are the smallest form of matter and cannot be created, destroyed or divided Postulates: Atoms of elements are identical; atoms can’t be created or destroyed; atoms combine in whole number ratios in a chemical reaction Discovered the electron “Plumpudding” Model Limitations Did not explain chemical behavior Did not include subatomic particles Did not explain deflection pattern of alpha particles as seen by Rutherford Could not explain why electrons do not crash into the nucleus No modification of atom model Discovered the proton and nucleus. Atoms contain mostly empty space. Discovered the neutron Electrons are in orbits of differing energy levels. Does not explain the behavior of other elements just hydrogen Electron cloud and dual nature of electrons Still doesn’t explain the exceptions Location Nucleus Nucleus outside the nucleus Charge 1+ 0 1- 14 6 11/3/14 C Electron cloud Mass 1 1 0 or 1/1840 Nuclear Symbol Carbon-14 Protons: Neutrons: Atomic Number: Mass Number: No new model/neutron later add to nucleus Identify the proton, electron, neutron in the model below. Parts of an Atom (8th Grade Review) Sub-atomic Particle Proton (p+) Neutron (n°) Electron (e-) Model ____6____ ____8____ ____6____ ____14___ SCIE_CHEM_ATOMIC_MAT_STUDYBUDDYTE_AL electron + + neutron proton What is the atomic number of the above models? 2 What is the atomic mass? 4.00 amu What element is this? Helium copyright © 2014 CFISD 1 Complete the Venn diagram below stating similarities and differences in the numbers of the subatomic particles for the same element. #p≠#e Same # proton and neutron Cation (+) Anion (-) Magnesium-25 25 12𝑀𝑔 Magnesium-24 24 12𝑀𝑔 Magnesium ion 24 2+ 12𝑀𝑔 #p=#e Same # proton and electrons No charge Different # of neutrons Types of Nuclear Radiation Type Composition Two protons and two neutrons High energy electron Alpha Beta Gamma Energy Reaction Type Fission Fusion Symbol 4 α or 2𝐻𝑒 β or 0 −1𝑒 0 0𝛾 Penetration/Energy Reaction Example Blocked by paper, low energy Blocked by wood, medium energy Blocked by lead or concrete, high energy Definition 149 62𝑆𝑚 → 145 60𝑁𝑑 165 61𝑃𝑚 → 0 −1𝑒 9 4𝐵𝑒 + 42𝐻𝑒 + 165 62𝑆𝑚 → 49𝐵𝑒 + 00𝛾 Diagram Uses Splitting of a nucleus into smaller fragments Fuel for nuclear power plants Combining nuclei to produce a nucleus of greater mass Energy source for nuclear bombs Reaction that occurs in the sun Balance the following nuclear equations: 58 0 58 228 224 4 28𝑁𝑖 → −1𝑒 + 29𝐶𝑢 88𝑅𝑎 → 86𝑅𝑛 + 2𝐻𝑒 12 6𝐶 → 00𝛾 + 126𝐶 Element X has two common isotopes, 79X (50.7%) and 81X (49.3%). What is the average atomic mass of element X? Identify element X. Average atomic mass = (79)(0.507) + (81)(0.493) = 79.9 amu = Bromine (Br) Copper occurs naturally as Copper-63 and Copper-65. Which Based on the atomic mass on the periodic table, which isotope is more abundant? The average atomic mass of copper is 63.546 amu. Since the average is closer to Copper63, it can be assumed that Copper-63 is more abundant. Why can chemists not simply round off atomic mass to the nearest whole number and say that would be the mass of the most abundant isotope? If copper (63.546 amu) were rounded, the result would be copper-64, which does not exist. 11/3/14 SCIE_CHEM_ATOMIC_MAT_STUDYBUDDYTE_AL copyright © 2014 CFISD 2