Acid Base Quiz
advertisement

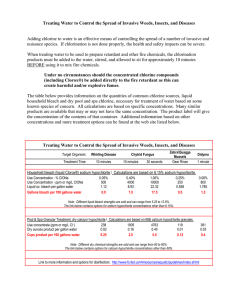
Acid Base Quiz- Name: 1. Don’t Mix Bleach with ___________, like: 2. __________ with Acid Toil Bowl Cleaners This mixture can result in toxic, potentially deadly fumes. 3. Bleach with vinegar Vinegar is a type of ____. Toxic chlorine vapor is produced. Don't mix chlorine bleach with any acid. 4. Bleach with ____________ Toxic, potentially lethal vapors are produced. 5. Different ____________ of One Type of Product Don't mix different cleaners together. They may react violently, produce toxins, or become ineffective. 6. Highly Alkaline Products with Highly Acidic Products Acids and bases (alkalis) can react violently, presenting a splash hazard. Acids and bases are caustic and may cause chemical reactions. 7. Certain Disinfectants with ________________ Don't mix disinfectants with 'quaternary ammonia' listed as an ingredient with a detergent. The effectiveness of the disinfectant may be neutralized. 8. Chlorine bleach is sometimes called “sodium hypochlorite” or “hypochlorite.” You will encounter it in chlorine bleach, chlorinated disinfectants and cleaners, chlorinated scouring powder, mildew removers, and toilet bowl cleaners. Do not mix products together. Do not mix them with ammonia or vinegar. Name 3 common acids or bases: 9. 10. 11. 12. What is the difference between an acid and a base? Acid/Base Lab Name: Define the following using your notes or your book: 1. 2. 3. 4. 5. 6. AcidBaseAmphotericNeutralizationpHSalt Using the Litmus paper and forceps, give the pH for the following substances: Step 1: Take a strip of litmus paper and place in forceps. Step 2: Dip one end of the litmus paper in the liquid and compare it to the side of the litmus paper vial. Match the color of the litmus paper to the same color on the vial. The corresponding number on the vial is the correct pH. Record the pH number below. pH 7. Equate Antacid tablets- ____________ 8. Tums Regular strength tablets-_________ 9. Vinegar-______________ 10. Water-____________ 11. Ammonia-___________ 12. Clorox Bleach__________ Comparison: _____________________ _____________________ _____________________ _____________________ _____________________ _____________________ 13. Looking at Figure 25.11 in your text, compare your values from 7 – 11. List a substance with a similar pH from the text under “Comparison” above.